Abstract
Tendinopathy is a common overuse injury with limited preventive options. This study evaluated a novel topical metformin lotion (ML) for its ability to prevent Achilles tendinopathy induced by intensive treadmill running (ITR) in mice. ML at concentrations of 3% and 6% was topically applied daily to the skin overlying the Achilles tendons for four weeks prior to the initiation of ITR. After the four-week topical ML pretreatment period prior to ITR induction, blood samples were collected for ELISA-based analysis of inflammatory markers (HMGB1, IL-1β, and PGE2), and tendons were examined using histological and immunofluorescent methods. ML inhibited the release of HMGB1 from cell nuclei into the tendon matrix, reduced serum levels of HMGB1, IL-1β, and PGE2, and exerted anti-degenerative effects by decreasing chondroid metaplasia, lowering the number of rounded cells, and enhancing collagen fiber organization. These beneficial effects of ML appeared to be mediated through increased AMPK activity, reduced TGF-β1 expression, decreased myofibroblast presence, lower collagen III levels, and elevated collagen I production. In conclusion, ML effectively prevents tendinopathy development, primarily by inhibiting HMGB1 and activating AMPK. These findings suggest that ML may serve as a convenient, effective, and non-invasive strategy to prevent tendinopathy in at-risk populations—such as athletes and military personnel—while minimizing systemic side effects.
Similar content being viewed by others
Introduction
Tendinopathy is a debilitating condition characterized by pain and inflammation in the affected tendon. It affects millions of individuals in occupational, athletic and military settings. The economic impact of tendinopathy is significant worldwide due to high treatment costs and productivity loss from reduced functional capacity1,2. The primary cause of tendinopathy is the overuse of tendons through repetitive movements3. The military population is particularly susceptible to tendinopathy due to the intensive and repetitive physical training required4. The Achilles tendon, the body’s largest tendon, is particularly susceptible to overuse-induced tendinopathy.
Despite the high prevalence of tendinopathy due to mechanical overuse, established preventive strategies remain scarce in the literature. While balance training has been suggested as a preventive intervention, tendinopathy still frequently develops, even in asymptomatic athletes with underlying tendon abnormalities5,6,7. Similarly, nutritional supplementation with agents such as curcumin and vitamin C has been proposed, but its protective benefits remain unverified8,9. Given the lack of reliable, evidence-based approaches for tendinopathy prevention, there is a critical need to develop novel, safe, and effective strategies to mitigate tendon injury risk, particularly in high-risk populations such as athletes and military personnel.
Inability to resolve inflammation is thought to be a major factor in early-stage tendinopathy development, with recent studies reporting elevated levels of inflammatory cells and markers in tendinopathic tendons10,11. Chronic inflammation may persist, leading to degenerative changes in the tendon over time12,13. Indeed, chronic inflammation is recognized as a hallmark of Achilles tendinopathy and tendon rupture, as evidenced by studies of Achilles tendon biopsies from symptomatic patients with mid-portion tendinopathy or rupture13. Abnormally high levels of inflammatory markers, such as cyclooxygenase-2 (COX-2) and prostaglandin E2 (PGE2), have been observed in mechanically overloaded mouse tendon cells in our previous studies14,15. In addition to provoking tendon inflammation, elevated COX-2 and PGE2 levels may drive aberrant differentiation of tendon stem cells into non-tenocytes, contributing to tendinopathy development15. Targeting chronic inflammation in the tendon matrix caused by mechanical overuse with pharmaceutical interventions may be an effective strategy to prevent Achilles tendinopathy.
High mobility group box 1 (HMGB1), a potent inflammatory mediator, plays a crucial role in various inflammatory diseases16,17. It has also been implicated in tendinopathy pathogenesis11,18,19,20,21,22. In injured tissues, this nuclear protein is released from the nucleus to the cytosol, plasma membrane, and extracellular space, where it triggers inflammation23. Elevated levels of extracellular HMGB1 have been observed in human tendinopathic tendons and are known to regulate inflammatory cytokines and matrix remodeling19. Our previous studies have demonstrated that HMGB1 can be released into the tendon extracellular matrix (ECM) and initiate an inflammatory cascade in response to mechanical overuse, as shown in a mouse Achilles tendinopathy model induced by intensive treadmill running (ITR)21,22. These findings suggest that HMGB1 plays a pivotal role in inflammation and indicate that blocking HMGB1 could have therapeutic potential for preventing inflammatory diseases, including tendinopathy.
Metformin (Met), an FDA-approved oral drug for diabetes treatment, is known to inhibit HMGB1 activity24. Studies have shown that Met has anti-inflammatory effects in various conditions, including cardiovascular disease, kidney disease, cancer, and neurodegenerative disorders25,26. In inflammatory diseases, damaged mitochondrial biogenesis can promote inflammatory macrophage responses, thereby compromising inflammation control by normal mitochondrial function27. Consequently, Met may serve as an effective therapeutic agent for inflammatory conditions such as tendinopathy.
Given that metformin is an FDA-approved drug with a well-characterized safety profile in humans, its repurposing in a topical formulation presents a highly translatable approach for clinical prevention of tendinopathy. The development of a non-invasive, locally targeted metformin lotion may overcome the limitations of systemic drug delivery, offering a practical, low-risk, and effective strategy for at-risk populations. These include athletes, military personnel, and workers in physically demanding occupations, who currently lack reliable pharmaceutical options for tendinopathy prevention.
Typically, Met is administered orally, which can lead to systemic side effects in the stomach, liver, and kidneys28 and is less effective on targeted peripheral tissues like tendons. Our previous studies showed that intraperitoneal (IP) injection of Met in mice prevented tendinopathy development in a mechanical overloading model22. However, IP injection is not ideal for humans due to systemic side effects. In this study, we developed a Met lotion (ML) as a novel transdermal drug to deliver Met directly to the tendon area by topically applying the lotion over the Achilles tendon. Using our validated mouse intensive treadmill running (ITR) Achilles tendinopathy model, we examined the preventive effects of ML on tendinopathy development. Our findings indicate that ML application prevents tendinopathy by reducing inflammation and tissue degeneration. The underlying mechanisms include inhibition of HMGB1 release, downregulation of transforming growth factor-β1 (TGF-β1) and α-smooth muscle actin (α-SMA), and activation of phospho-AMP-activated protein kinase (p-AMPK).
Results
Met lotion reaches the Cmax of metformin in blood more rapidly than oral administration
Pharmacokinetics study in serum samples showed that as a transdermal drug, ML delivered Met to blood faster and more effective than oral administration after administration of the same dose of Met. The concentration of Met in the serum samples of the mice was increased quickly after ML administration and reached maximum concentration (Cmax) of 635.5 µg/ml at 4-hour (Supp Fig. S1). The Cmax of Met was achieved to only 566.6 µg/ml in mouse blood in 5 h post-administration of oral Met. Statistical analysis revealed that the Met concentration in the Met lotion group was significantly different from that in the oral group at 2 and 4 h (p < 0.05). Met was not detectable in the DD water or Met lotion vehicle groups; therefore, their results are not shown in (Supplementary Fig. S1).
Met lotion reduces ITR-induced inflammation
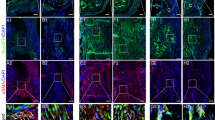
Immunofluorescence staining without Triton X-100 treatment (Fig. 1) showed elevated HMGB1 levels in the tendon matrix of ITR-subjected mice, indicating that mechanical overuse induced HMGB1 release from the cell nuclei (red fluorescence in Fig. 1a–d) into the tendon matrix. In contrast, HMGB1 levels were significantly lower in the tendon matrix of ML-treated mice (Fig. 1e–l), suggesting that ML inhibits HMGB1 release. Semi-quantification confirmed these findings, with approximately 64.3% positive staining in ITR-only tendons compared to 11.9% and 2.1% in tendons treated with 3% and 6% ML, respectively (Fig. 1m).
Met lotion inhibits ITR-induced HMGB1 release into the tendon matrix in tissue sections analyzed without Triton X-100 treatment. Without Triton X-100 treatment, only HMGB1 released to tendon matrix is positively stained. The results indicate that ITR induces HMGB1 release into the tendon matrix (red fluorescence: b–d), which is inhibited by ML (e–l). Semi-quantitative analysis confirms these results, showing that ML applications significantly decrease positively stained cells in the matrix, with 3% ML showing a 5.9-fold decrease and 6% ML showing minimal positive staining (m). c,g,k: Merged images of(a,b,e,f; and i,j respectively. d,h,l are enlarged images of c,g,k respectively. m: Semi-quantitative results from 45 images (15 sections from 5 mice per group) are presented as mean ± SD (n = 5). ML: Met Lotion. Scale bars: 50 μm (yellow); 15 μm (white).
Further experiments with Triton X-100 treatment (Fig. 2) showed few cells with nuclear HMGB1 staining in ITR tendons (Fig. 2a–d), whereas ML-treated tendons (Fig. 2e–l) exhibited high levels of nuclear HMGB1 (yellow arrows in Fig. 2h, l). Semi-quantification indicated approximately 13.2% nuclear HMGB1 in ITR tendons, compared to 68.7% and 71.1% in ML-treated tendons (Fig. 2m).
Met lotion inhibits ITR-induced HMGB1 release into the tendon matrix in tissue sections analyzed with Triton X-100 treatment. In tendon tissues treated with Triton X-100, which allows staining to detect HMGB1 both in the tendon matrix and within cell nuclei, staining results show that high levels of HMGB1 remain localized in the cell nuclei of ML-treated tendons (e–l), whereas ITR releases most of HMGB1 from nuclei (b–d). Semi-quantitative analysis of immunostaining results shows a 4-fold increase in positively stained nuclei in ML-applied samples compared to ITR-only samples (m). c,g,k: Merged images of a,b;e,f; and i,j respectively. d,h,l are enlarged images of c,g,k, respectively. m: Semi-quantitative results from 45 images (15 sections from 5 mice per group) are presented as mean ± SD (n = 5). ML: Met Lotion. Scale bars: 50 μm (yellow); 15 μm (white).
ELISA results corroborated these findings, showing significant reductions in serum HMGB1 levels in ML-treated mice after 4 weeks of ITR. HMGB1 levels were 28.6 ng/ml in ITR-only mice, which decreased to 13.7 ng/ml and 7.9 ng/ml in mice treated with 3% and 6% ML, respectively (Fig. 3A). Additionally, ML application reduced other inflammatory markers, IL-1β and PGE2, in serum samples (Fig. 3B, C). IL-1β levels were 777.8 pg/ml in ITR-only mice but dropped to 578.6 pg/ml and 236.9 pg/ml in mice treated with 3% and 6% ML, respectively (Fig. 3B). PGE2 levels were 1567.6 pg/ml in the ITR-only mice but decreased to 1247.2 pg/ml and 880.4 pg/ml in 3% and 6% ML-treated mice, respectively (Fig. 3C).
Met lotion reduces levels of inflammatory markers in mouse serum. ML decreases HMGB1 levels significantly in the serum of ITR-induced mice in a concentration-dependent manner (A). HMGB1 levels in ITR-only mice are twice as high as in mice treated with 3% ML and 3.6-fold higher than in mice with 6% ML application, with 6% ML showing greater efficacy (A). IL-1β levels in ITR mice also decrease significantly with ML application, with 6% ML being more effective than 3% (B). Similarly, ML application reduces PGE2 levels in the serum of ITR mice, with 6% ML more effective than 3% (C). The results are represented by mean ± SD from three different mice of each group (n = 3). ML: Met Lotion.
Met lotion inhibits ITR-induced tendon degeneration associated with failed remodeling
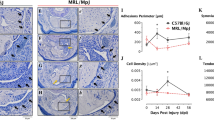
H&E staining revealed ITR-induced tendon degeneration (Fig. 4a–d), characterized by disrupted enthesis (Ent) structure (black arrows in Fig. 4b), disorganized collagen fibers (green arrows in Fig. 4c), numerous chondrocyte-like cells (blue arrows in Fig. 4d), and many round-shaped cells (yellow box areas in Fig. 4c, d). ML application mitigated these changes, resulting in well-organized fibrocartilage in the enthesis area (green box areas in Fig. 4e, f, i, j) and fewer round-shaped cells (yellow box areas in Fig. 4g, h, k, l). Semi-quantification showed 21.5% round-shaped (chondrocyte-like) cells in ITR tendons, compared to 2.2% and 1.1% in 3% and 6% ML-treated tendons, respectively (Fig. 4m).
Met lotion improves tendon structure by inhibiting degeneration associated with failed remodeling. ITR induces tendon degeneration, characterized by thinner collagen fibers, reduced enthesis size, and an increase in round-shaped chondrocyte-like cells (a–d). ML applications mitigate these degenerative changes, resulting in well-organized collagen fibers in the enthesis area, larger fibrocartilage tissue, and fewer chondrocyte-like cells in tendon tissues (e–l). Semi-quantitative analysis shows a significant decrease in chondrocyte-like cells with a 11-fold and 21-fold reduction in tendons with 3% and 6% ML applications, respectively (m). b,f,j: Enlarged green box areas from images (a,e,i; c,g,k: Enlarged blue box areas from images a,e,i; d,h,l: Enlarged yellow box areas from images c,g,k.m: Semi-quantitative results from 45 images (15 sections from 5 mice per group) are presented as mean ± SD (n = 5). ML: Met Lotion. Scale bars: 500 μm (black); 200 μm (blue); 50 μm (white); 12.5 μm (yellow).
Met lotion prevents ITR-induced tendon degeneration associated with failed remodeling
SFO and FG staining confirmed tendon degeneration in ITR mice (Fig. 5a–d), characterized by thinner collagen fibers (red arrows in Fig. 5b) and a reduced enthesis size, defined by its width in the sagittal plane of the Achilles tendon (pink arrow line in Fig. 5b). This reduction in enthesis size was further validated by semi-quantitative analysis (Fig. 5m). In addition, numerous round-shaped, chondrocyte-like cells were observed within the tendon (yellow arrows in Fig. 5c, d). ML application (Fig. 5e–l) preserved collagen matrix structure and increased fibrocartilage size (white arrow line in Fig. 5f, j), with more elongated cells (pink arrows in Fig. 5g, h, k, l).
Met lotion prevents tendon degeneration during failed remodeling by enhancing collagen organization, promoting cell elongation, and enlarging enthesis size. ITR induces degenerative changes such as thinner collagen fibers, reduced enthesis size, and an increase in round-shaped chondrocyte-like cells (a–d). ML application inhibits these degenerative changes, resulting in better organized collagen fibers in the enthesis area, larger fibrocartilage tissue, and more elongated cells in the tendon tissues (e–l). b,f,j:Enlarged yellow box areas from images a,e,i. c,g,k: Enlarged red box areas from images a,e,i. d,h: Enlarged pink box areas from images c,g,k. m: Semi-quantitative results for enthesis size are reported as mean ± SD, based on five mice per group (n = 5). ML: Met Lotion. Scale bars: 500 μm (black); 100 μm (yellow); 50 μm (pink); 12.5 μm (red).
Trichrome staining showed loose collagen fibers in ITR tendons (blue staining in Supp Fig. S2a–d) and a smaller enthesis (yellow arrow line in Supp Fig. S2b) compared to the tendons treated with ML, which exhibited red-stained collagen fibers (green arrows in S2 Fig. g, h, k, l), suggesting the presence of dense, mature collagen fibers. Additionally, a larger enthesis was observed in the ML application group as well (white arrow line in Supp Fig. S2j).
Met lotion inhibits ITR-induced collagen III production
Picro Sirius Red staining under polarized light microscopy revealed high collagen III levels in ITR tendons (green fluorescence in Supp Fig. S3j–l), while ML-application tendons showed collagen I dominance (red fluorescence in Supp Fig. S3m–r). Although all groups displayed red-stained collagen fibers under bright light (Supp Fig. S3a–i), ITR tendons were disorganized (black arrows in Supp Fig. S3a, c), whereas ML-application tendons maintained organized structures (Supp Fig. S3d–i).
Met lotion prevents ITR-induced tendon disorganization
Histological analysis revealed that ITR induces tendon degeneration and scar tissue formation, both of which are key characteristics of tendinopathy. ML application effectively prevents tendinopathy development by inhibiting tendon degeneration associated with failed remodeling.
Collagen II and SOX-9 are well-established markers of chondrocytes, while recent studies suggest that elevated α-SMA and collagen III levels contribute to the formation of loose collagen fibers, which play a critical role in scar tissue formation. TGF-β1 has been implicated in fibrotic scar development, a process that can lead to degenerative tendinopathy29. Additionally, AMPK is known to regulate inflammatory signaling and reduce scar tissue formation30.
To further support our histological findings, we investigated the effect of ML on tendinopathy prevention through immunostaining. Immunostaining showed that collagen II was present in ITR tendons, with more than 47% positive staining (Fig. 6a–c, j). This decreased to 20.7% and 16.0% in tendons with 3% and 6% ML application, respectively (Fig. 6d–i, j). Immunostaining also showed 38.1% Sox-9 positive cells in ITR tendons (Fig. 7a–d, m), compared to 5.0% and 2.4% in tendons applied with 3% and 6% ML, respectively (Fig. 7e–l, m).
Met lotion reduces ITR-induced collagen II formation. ML application decreases collagen II expression (d–i), which was elevated by ITR (a–c). Semi-quantitative analysis shows a 1.8-fold and 2.9-fold decrease in collagen II with 3% and 6% ML application, respectively (j). b,e,h: Enlarged green box areas from images a,d,g. c,f,i: Enlarged yellow box areas from images b,e,h. j: Semi-quantitative results from 45 images (15 sections from 5 mice per group) are presented as mean ± SD (n = 5). ML: Met Lotion. Scale bars: 500 μm (white); 200 μm (yellow); 50 μm (green).
Met lotion reduces SOX-9 expression in tendons. ML application at 3% and 6% significantly decreases SOX-9 expression (e–l,m) compared to ITR alone (a–d,m), showing a 7.6-fold and 15.5-fold reduction with 3% and 6% ML application, respectively. b,f,j: Enlarged green box areas from images a,e,i. c,g,k: Enlarged orange box areas from images b,f,j. d,h,l: Enlarged yellow box areas from images c,g,k. m: Semi-quantitative results from 45 images (15 sections from 5 mice per group) are presented as mean ± SD (n = 5). ML: Met Lotion. Scale bars: 200 μm (white); 100 μm (orange); 50 μm (yellow); 12.5 μm (blue).
TGF-β1 expression was elevated in ITR tendons, with 63.2% positive staining (Fig. 8a–d, m). This decreased to 8.9% and 2.7% in tendons applied with 3% and 6% ML, respectively (Fig. 8e–l, m).
Met lotion decreases TGF-β1 expression in tendons. ML application significantly decreases TGF-β1 expression in ITR tendons in a concentration-dependent manner, with a 7.2-fold and 23.3-fold reduction for 3% and 6% ML application, respectively (e–l,m), compared to ITR tendons (a–d,m). m: Semi-quantitative results from 45 images (15 sections from 5 mice per group) are presented as mean ± SD (n = 5). ML: Met Lotion. Scale bars: 500 μm (white); 200 μm (yellow); 100 μm (green); 50 μm (orange).
Immunostaining for α-SMA revealed that ITR increased α-SMA levels in mouse tendon tissues (Supp Fig. S4a–d). Approximately 63% of cells in ITR-treated tendons were positively stained for α-SMA (Supp Fig. S4a–d, m), while only about 2.6% and 3.2% of cells in tendons applied with 3% and 6% ML, respectively, expressed α-SMA (Supp Fig. S4e–l, m).
Additionally, immunostaining for p-AMPK showed that ML application enhanced p-AMPK expression in ITR tendons. Approximately 52.9% and 58.6% of cells in tendons applied with 3% and 6% ML, respectively, were positively stained for p-AMPK (Supp Fig. S5c–f, g), compared to only 3.5% of cells in ITR tendons (Supp Fig. S5a, b,g).
Discussion
Tendinopathy is a common and debilitating overuse injury with limited preventive options. In this study, we evaluated the preventive effects of a novel topical metformin lotion (ML) in a mouse model of mechanically induced Achilles tendinopathy. ML application significantly reduced tendon inflammation and degeneration, effectively preventing tendinopathy development. Specifically, ML lowered serum levels of inflammatory markers (HMGB1, IL-1β, and PGE2), reduced the presence of chondrocyte-like cells, and improved collagen fiber organization. These effects were associated with increased AMPK activity, reduced TGF-β1 expression, decreased myofibroblast presence, lower collagen III levels, and enhanced collagen I production. This non-invasive therapeutic approach holds promise for preventing tendinopathy in at-risk human populations, addressing a critical gap in current prevention strategies.
Exercise-based programs—including Achilles tendon stretching, plantar flexion strengthening, and balance training—have been implemented as preventive strategies against tendinopathy development5,6. However, randomized controlled trials evaluating these interventions have not consistently demonstrated significant reductions in the incidence of Achilles or patellar tendinopathy31,32,33. While eccentric and concentric loading exercises are well-established treatments for symptomatic tendinopathy, there is growing interest in their potential preventive applications, particularly among high-risk populations such as athletes and military personnel. Targeted loading strategies—such as progressive eccentric or isotonic training—are being investigated for their ability to promote tendon resilience by stimulating adaptive remodeling and improving load tolerance. Although the clinical evidence supporting their use for primary prevention is still limited compared to therapeutic use, preseason conditioning and neuromuscular training programs incorporating these modalities have shown promise in reducing tendinopathy incidence in sports involving repetitive lower limb loading34,35.
Avoiding medications like fluoroquinolones, which are known to be associated with tendinopathy risk, is also recommended36. Nutrients with antioxidant and anti-inflammatory properties, such as vitamin C, vitamin D, and curcumin, are gaining attention as potential preventive measures9,37. Animal studies have shown that curcumin may positively affect tendon healing38,39; however, further research is needed to confirm its efficacy, optimize bioavailability, and determine appropriate dosing in humans. Additionally, these supplements are generally aimed at reducing symptoms or aiding treatment in existing cases of tendinopathy rather than serving as preventive measures. Currently, there are no pharmaceuticals specifically intended for the prevention of tendinopathy.
Metformin’s anti-inflammatory, antioxidant, and anti-fibrotic properties make it a promising approach for the prevention of tendinopathy. Our previous studies using IP injection demonstrated that metformin inhibits HMGB1 release and prevents tendinopathy in our ITR-induced tendinopathy model in mice22exerts anti-inflammatory and anti-senescent effects in aging tendons, thereby mitigating tendon inflammation and degeneration40 and enhancing healing in a tendon injury model41. However, due to the potential systemic side effects of injected metformin (e.g., nausea, vomiting, diarrhea, and abdominal discomfort) and the risk of more serious conditions like lactic acidosis in certain patients28,42,43, we have shifted to transdermal ML application to mitigate these systemic risks.
As demonstrated in our study, ML effectively prevents tendinopathy through multiple mechanisms, including blocking HMGB1 release (an inflammatory marker), reducing TGF-β1 expression (a fibrotic tissue marker), decreasing myofibroblast proliferation, as evidenced by low α-SMA expression, and activating AMPK, which regulates inflammatory signaling. By targeting these pathways, ML not only mitigates inflammation but also prevents failed tendon remodeling, a key contributor to degenerative tendinopathy. Disrupted remodeling is characterized by excessive scar tissue formation, collagen disorganization, and persistent inflammatory signaling, all of which can impair the structural integrity and function of the tendon. This targeted, localized approach effectively addresses the inflammatory and degenerative pathways involved in failed tendon remodeling, while minimizing systemic side effects. The formulation’s controlled-release properties enable sustained local drug presence, enhancing therapeutic efficacy and reducing the need for frequent dosing44. Importantly, this study also demonstrates the translational potential of ML as a preventive therapy for tendinopathy in humans. The topical formulation leverages the established safety of metformin while minimizing systemic exposure, a key consideration for clinical application. Given the high burden of tendinopathy in physically active populations and the absence of approved preventive pharmaceuticals, ML could represent a first-in-class solution that is both accessible and scalable for widespread use. Furthermore, its ease of use and non-invasive administration enhance its suitability for longitudinal deployment in sports, military, and occupational health settings.
Blocking HMGB1 release triggered by mechanical overuse may be a critical step in preventing tendinopathy development, as clinical samples of early-stage tendinopathy have shown elevated HMGB1 levels19,45,46. High HMGB1 levels can initiate tendon inflammation and subsequent degeneration. Released HMGB1 can also attract inflammatory cells, such as macrophages, which in turn may release other inflammatory cytokines, including IL-1β, IL-6, and IL-3319,47,48. Our findings suggest that ML application could inhibit HMGB1 release and reduce levels of other inflammatory mediators, such as IL-1β and PGE2, thereby controlling the inflammatory cascade.
We have also demonstrated that ML application reduces the expression of collagen II and SOX-9 (chondrogenic markers) as well as TGF-β1, α-SMA, and collagen III (fibrotic tissue markers), while increasing collagen I production and activating AMPK. The presence of chondrocyte-like cells (SOX-9 and collagen II-positive cells) in ITR tendons is considered a key characteristic in the development of tendinopathy21.
TGF-β1 is considered a key mediator of fibrosis, promoting tendon adhesion and scar formation, and elevated α-SMA expression has been associated with scar formation49,50,51. In our recent work, we demonstrated that metformin injections reduced SOX-9 and collagen II expression, decreased TGF-β1 expression, inhibited the migration of α-SMA+ cells to the wound area, decreased collagen III accumulation, and increased collagen I production and p-AMPK levels in a tendon wound healing mouse model41.
AMPK is a well-established regulator of whole-body energy homeostasis52. In vivo studies have demonstrated that AMPK is activated in skeletal muscle during treadmill exercise in an intensity-dependent manner53. Furthermore, AMPK activation has been shown to promote autophagy and enhance mitochondrial function54. In the present study, ML was found to activate AMPK, suggesting its potential to improve mitochondrial metabolism. This may contribute to the prevention or mitigation of tendinopathy by helping support cellular energy demands, promote tissue repair, and reduce degenerative changes associated with tendinopathy. However, the precise molecular mechanisms by which ML exerts these effects remain to be elucidated.
One limitation of this study is that the effects of ML were evaluated only in female mice. In future studies, we will include both male and female mice, as differences in skin thickness between sexes may influence transdermal absorption. Another limitation is that we assessed only the preventive effects of ML using a 4-week treadmill running model, which primarily induces early-stage tendinopathy, as demonstrated in this study. Additionally, the treadmill experiments did not include an ITR plus vehicle group, raising the question of whether lotion excipients might influence the Achilles tendon. However, in a separate study, we found that histological outcomes for the ITR plus vehicle group were similar to those of the ITR group, suggesting no additional effect from the vehicle. This finding aligns with expectations, as the excipients in our Met lotion are FDA-approved inert substances and are unlikely to penetrate the Achilles tendon due to their large molecular size and physicochemical properties.
Additionally, sample sizes in some experiments of this study were relatively small, and no functional evaluations (e.g., mechanical testing) or confirmatory analyses (e.g., transcriptomics, proteomics, or metabolic pathway studies) were performed to further investigate the mechanisms by which ML may prevent tendinopathy. These limitations will be addressed in future studies.
Due to the small size of mice and limited blood volume, pharmacokinetic (PK) samples were not collected longitudinally from the same animals, which we also acknowledge as a limitation.
Finally, metformin is known to enhance mitochondrial biogenesis—a process that increases mitochondrial mass and promotes metabolic homeostasis by facilitating the turnover of healthy mitochondria and clearance of damaged ones55,56. ML may act through a similar pathway, which warrants further investigation. The use of tendon-specific AMPK knockout models57 will be valuable in exploring these mechanisms.
Significance and clinical relevance
This study positions ML as a promising preventive intervention for tendinopathy by demonstrating its ability to block HMGB1 release, suppress TGF-β1 expression, reduce myofibroblast presence, and activate AMPK. These findings support the potential of ML as a convenient, effective, and non-invasive strategy for preventing tendinopathy in at-risk populations, including athletes, military personnel, and individuals engaged in physically demanding occupations.
Materials and methods
Animals
All animals were housed under standard laboratory conditions in the Division of Laboratory Animal Resources (DLAR) facility at the University of Pittsburgh, with a controlled temperature of 22 °C, relative humidity maintained between 40% and 60%, and a 12-hour light/dark cycle. Animals had ad libitum access to food and water. All animals were allowed to acclimate to the vivarium environment for a minimum of 7 days prior to the initiation of experimental procedures to reduce stress and ensure physiological stabilization. All procedures involving animals were approved by the IACUC (Protocol #22091904) and conducted in accordance with institutional guidelines, the National Research Council’s Guide for the Care and Use of Laboratory Animals, and the ARRIVE guidelines.
Materials
Metformin hydrochloride (Cat. #PHR1084, pharmaceutical secondary standard), glycerol (Cat. #15524), Vaseline® (Cat. #16415), paraffin oil (Cat. #18512), and sorbitan sesquioleate (Span 83; Cat. #S3386), all meeting analytical specifications of Ph. Eur. and BP, were purchased from Millipore Sigma (Burlington, MA).
Met lotion preparation
ML was produced using a proprietary, optimized formulation process. Both 3% and 6% (w/w) ML formulations were white, homogeneous lotions. Our pharmacokinetic testing confirmed that, when applied evenly on animal skin, the lotion effectively promoted Met diffusion through the skin and reached the Achilles tendon, supporting its potential as a controlled release formulation58.
Pharmacokinetic study of metformin administered via oral gavage and topical lotion application
For oral administration, Met was dissolved with deionized distilled (DD) water to make a Met-solution with the concentration of 20 mg/ml. Met-solution (160 µl) was given into each mouse by an oral gavage with the dose of 3.2 mg/mouse (160 mg/kg body weight). For transdermal administration, 50 mg of the 3% ML was smeared on the skin over the Achilles tendon area of each leg, delivering a dose of 3 mg of Met to each mouse (equivalent to 160 mg/kg body weight). Three mice from each group were sacrificed at each specified time point (0, 1, 2, 3, 4, 5, 6, 7, 8, 9, and 10 h). The blood samples were collected from hearts of the mice and allowed to sit at room temperature for 1 h before centrifugation at 1000 × g for 15 min. The supernatant was separated from the red blood cell pellets and stored at -20 °C until analysis. The pharmacokinetics of Met administered orally or via ML was assessed by measuring serum Met concentrations at each time point post-administration, following our published protocol58. Identical procedures and assessment methods were used for both control groups: oral DD water and 0% Met lotion (vehicle).
Mouse treadmill running protocol and metformin lotion application
This study used 30 female C57BL/6J mice, each 3 months old, divided into three groups of 10 mice each. Achilles tendinopathy was induced using an ITR protocol21. During the first week, mice ran at a speed of 15 m/min for 15 min per day, 5 days a week. This was followed by running at the same speed for 3 h per day, 5 days a week, for 4 weeks. Group 1 (ITR only): Mice followed the ITR protocol without treatment. Group 2 (ITR + 3% ML): Mice received 50 mg of 3% ML applied to the skin over the Achilles tendon of each leg before daily treadmill running session. Group 3 (ITR + 6% ML): Mice received 50 mg of 6% ML applied to the same area on each leg before daily treadmill running session. Note that the selection of the two ML concentrations was based on data from our pharmacokinetic study (see Supp Fig. S1 for details).
All mice were monitored daily and sacrificed after the 4-week experimental period by inhalation of carbon dioxide followed by cervical dislocation and thoracotomy to verify the death of mice. Blood samples collected from the heart were used to measure levels of inflammatory mediators HMGB1, interleukin-1β (IL-1β), and PGE2, using ELISA kits. Achilles tendon tissues were harvested for histological analysis using histochemical and immunofluorescence staining.
Measurement of HMGB1, IL-1β, and PGE2 in mouse serum samples
Concentrations of HMGB1, IL-1β, and PGE2 in mouse serum were measured using respective ELISA kits, following the manufacturer’s instructions (HMGB1: Cat. #MBS2021855, MyBioSource, San Diego, CA, USA; IL-1β: Cat. #ab108866, Abcam, Boston, MA, USA; PGE2: Cat. #MBS266212, MyBioSource, San Diego, CA, USA). Blood was collected from the hearts of the mice and allowed to sit at room temperature for 1 h before centrifugation at 1000 × g for 15 min. The supernatant was separated from the red blood cell pellets and stored at -20 °C until analysis.
Histochemical staining for structural analysis of mouse tendon tissues
The hind legs were dissected from the knee and de-skinned. Tissue samples were fixed with 4% paraformaldehyde overnight at room temperature. Fixed tissues were then treated with Cal-Ex II Fixative/Decalcifier at room temperature for one week (Cat. #CS511-1D; Fisher Scientific, Pittsburgh, PA). Decalcification completion was verified by chemical testing: 1 ml of decalcified sample solution was mixed with 1 ml of 5% ammonium hydroxide and 1 ml of 5% ammonium oxalate solution, then left for 30 min. A clear solution indicated completion of decalcification.
The decalcified tissues were embedded in paraffin and sectioned longitudinally in the sagittal plane of the Achilles tendon into 5 μm-thick slices. These tissue sections were serially collected and numbered sequentially from the surface inward. Specific section numbers were designated for each stain to ensure consistent sampling across all mice. For example, sections #1, #15, #30, and #45 were stained with H&E; #2, #16, #31, and #46 with Safranin O & Fast Green; #3, #17, #32, and #47 with Masson’s Trichrome; and #4, #18, #33, and #48 with Picrosirius Red. Trichrome staining (Cat. #ab150686) and Picrosirius Red staining (Cat. #ab150681) were performed using kits from Abcam (Waltham, MA, USA). Stained tendon sections were examined using a light microscope (Nikon Eclipse TE2000-U) or analyzed under a polarized light microscope (Nikon).
Immunofluorescent staining of mouse tendon tissue sections
For immunofluorescent staining, decalcified mouse Achilles tendon-containing hind legs were embedded in O.C.T. compound (Sakura Finetek USA Inc., Torrance, CA) within disposable molds and frozen at -80 °C. Cryostat Sect. (5 μm thick) were obtained at -25 °C and allowed to air dry overnight at room temperature. Sections were fixed in 4% paraformaldehyde for 15 min and washed with PBS. The tissue sections with the same number from each group were used for the same marker staining. For staining HMGB1, p-AMPK, and SOX-9, sections were permeabilized with 0.1% Triton X-100 at room temperature for 30 min, then washed with PBS. Note that Triton X-100 was used to permeabilize cell membranes, allowing detection of HMGB1 within cell nuclei as well as the tendon matrix.
Sections were incubated overnight at 4 °C with primary antibodies: rabbit anti-HMGB1 (1:330; Cat. #ab18256, Abcam), rabbit anti-phospho-AMPK (1:500; Cat. #ab133448, Abcam), rabbit anti-SOX-9 (1:500; Cat. #ab185966, Abcam), rabbit anti-α-SMA (1:500; Cat. #ab124964, Abcam), rabbit anti-TGF-β1 (1:500; Cat. #ab215715, Abcam), or rabbit anti-collagen II (1:500; Cat. #ab34712, Abcam). The following day, sections were washed with PBS and incubated for 2 h at room temperature with a Cy3-conjugated goat anti-rabbit secondary antibody (1:500; Cat. #AP132C, Millipore, Burlington, MA). Sections were counterstained with DAPI and analyzed under a fluorescent microscope (Nikon, Eclipse TE2000-U).
Semi-quantification of positive staining and enthesis size
To achieve semi-quantitative results, three random images per tendon section were captured using a Nikon Eclipse TE2000-U microscope. A total of 45 images were analyzed in a blinded manner, with nine images taken from three sections per mouse, across 15 sections from five different mice per group. Images were manually inspected to identify positively stained cells and then processed using SPOT imaging software v5.3 (Diagnostic Instruments, https://www.spotimaging.com/software/). The percentage of positive staining was calculated in two ways: (1) by dividing the positively stained area by the total image area or (2) by dividing the number of positively stained cells by the total number of cells, which were stained either with hematoxylin (for histochemical staining) or DAPI (for immunofluorescent staining). The resulting percentages were multiplied by 100 and then averaged to determine the overall positive staining for each group.
Enthesis size, defined as the width of the Achilles tendon insertion in the sagittal plane, was measured using SPOT Imaging Software (version 5.3). For each section, three images were captured at 4×, 10×, and 40× magnifications to ensure accurate visualization of the enthesis. Three sections per mouse were analyzed, with five mice per group. Measurements from all images were averaged to obtain a single enthesis size per mouse, and group data were reported as mean ± SD for statistical analysis.
Number of animals used in this study and animal allocation
A total of 153 mice were used in this study. Of these, 123 mice were allocated for pharmacokinetic (PK) analysis—3 mice per time point across 10 time points (1–10 h) for each of 4 groups (oral Met, oral DD water, 0% Met lotion, and 3% Met lotion), along with 3 untreated controls (at 0 h time point). The remaining 30 mice were used for treadmill running experiments. For ELISA analysis of HMGB1, IL-1β, and PGE2 in serum, 9 mice from each treadmill running group (ITR, ITR + 3% ML, and ITR + 6% ML) were used. Within each group, 3 mice were allocated for HMGB1 measurement, 3 for IL-1β, and 3 for PGE2. For semi-quantitative histological analysis, 5 mice per group were included. From each mouse, 3 tissue sections were obtained, and 3 images per section were captured, yielding 9 images per mouse and a total of 45 images per group. Results represent the average values calculated from these 45 images. Note that only 5 mice per group were included in the histological analysis due to technical issues—some tendon samples were not properly embedded, sectioned, or stained.
Statistical analysis
Continuous data from ELISA analysis (HMGB1, IL-1β, and PGE2) were analyzed using one-way ANOVA followed by Fisher’s LSD test for multiple comparisons. For those remaining semi-quantification data, however, they were analyzed using non-parametric statistical methods due to the bounded nature of percentage data and potential deviations from normality. Comparisons among the three experimental groups (ITR, ITR + 3% ML, and ITR + 6% ML) were first evaluated using the Kruskal–Wallis test to determine overall group differences. When a significant group effect was observed, pairwise comparisons were performed using the Mann–Whitney U test with Bonferroni correction to identify specific differences between groups. Statistical significance was determined at a threshold of p < 0.05. All tests were conducted using standard statistical libraries.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Lipman, K., Wang, C., Ting, K., Soo, C. & Zheng, Z. Tendinopathy: injury, repair, and current exploration. Drug. Des. Devel. Ther. 12, 591–603. https://doi.org/10.2147/DDDT.S154660 (2018).
Report. Global Tendinopathy Market to 2030 - Insights, Epidemiology and Forecasts - https://ResearchAndMarkets.com. (2021).
Magnusson, S. P., Langberg, H. & Kjaer, M. The pathogenesis of tendinopathy: balancing the response to loading. Nat. Rev. Rheumatol. 6, 262–268. https://doi.org/10.1038/nrrheum.2010.43 (2010).
Owens, B. D. et al. Risk factors for lower extremity tendinopathies in military personnel. Orthop. J. Sports Med. 1, 2325967113492707. https://doi.org/10.1177/2325967113492707 (2013).
Kvist, M. Achilles tendon injuries in athletes. Sports Med. 18, 173–201. https://doi.org/10.2165/00007256-199418030-00004 (1994).
Kraemer, R. & Knobloch, K. A soccer-specific balance training program for hamstring muscle and patellar and Achilles tendon injuries: an intervention study in premier league female soccer. Am. J. Sports Med. 37, 1384–1393. https://doi.org/10.1177/0363546509333012 (2009).
Theodorou, A., Komnos, G. & Hantes, M. Patellar tendinopathy: an overview of prevalence, risk factors, screening, diagnosis, treatment and prevention. Arch. Orthop. Trauma. Surg. 143, 6695–6705. https://doi.org/10.1007/s00402-023-04998-5 (2023).
Noriega-Gonzalez, D. C. et al. Effect of vitamin C on tendinopathy recovery: A scoping review. Nutrients 14 https://doi.org/10.3390/nu14132663 (2022).
Burton, I. & McCormack, A. Nutritional supplements in the clinical management of tendinopathy: A scoping review. J. Sport Rehabil. 32, 493–504. https://doi.org/10.1123/jsr.2022-0244 (2023).
Rees, J. D., Stride, M. & Scott, A. Tendons–time to revisit inflammation. Br. J. Sports Med. 48, 1553–1557. https://doi.org/10.1136/bjsports-2012-091957 (2014).
Millar, N. L., Murrell, G. A. & McInnes, I. B. Inflammatory mechanisms in tendinopathy - towards translation. Nat. Rev. Rheumatol. 13, 110–122. https://doi.org/10.1038/nrrheum.2016.213 (2017).
Dakin, S. G., Dudhia, J. & Smith, R. K. Resolving an inflammatory concept: the importance of inflammation and resolution in tendinopathy. Vet. Immunol. Immunopathol. 158, 121–127. https://doi.org/10.1016/j.vetimm.2014.01.007 (2014).
Dakin, S. G. et al. Chronic inflammation is a feature of Achilles tendinopathy and rupture. Br. J. Sports Med. 52, 359–367. https://doi.org/10.1136/bjsports-2017-098161 (2018).
Yang, G., Im, H. J. & Wang, J. H. Repetitive mechanical stretching modulates IL-1beta induced COX-2, MMP-1 expression, and PGE2 production in human patellar tendon fibroblasts. Gene 363, 166–172. https://doi.org/10.1016/j.gene.2005.08.006 (2005).
Zhang, J. & Wang, J. H. Production of PGE(2) increases in tendons subjected to repetitive mechanical loading and induces differentiation of tendon stem cells into non-tenocytes. J. Orthop. Research: Official Publication Orthop. Res. Soc. 28, 198–203. https://doi.org/10.1002/jor.20962 (2010).
Andersson, U. & Tracey, K. J. HMGB1 is a therapeutic target for sterile inflammation and infection. Annu. Rev. Immunol. 29, 139–162. https://doi.org/10.1146/annurev-immunol-030409-101323 (2011).
Harris, H. E., Andersson, U. & Pisetsky, D. S. HMGB1: a multifunctional alarmin driving autoimmune and inflammatory disease. Nat. Rev. Rheumatol. 8, 195–202. https://doi.org/10.1038/nrrheum.2011.222 (2012).
Millar, N. L., Murrell, G. A. & McInnes, I. B. Alarmins in tendinopathy: unravelling new mechanisms in a common disease. Rheumatol. (Oxford). 52, 769–779. https://doi.org/10.1093/rheumatology/kes409 (2013).
Akbar, M. et al. Targeting danger molecules in tendinopathy: the HMGB1/TLR4 axis. RMD Open. 3, e000456. https://doi.org/10.1136/rmdopen-2017-000456 (2017).
Thankam, F. G. et al. Association of inflammatory responses and ECM disorganization with HMGB1 upregulation and NLRP3 inflammasome activation in the injured rotator cuff tendon. Sci. Rep. 8, 8918. https://doi.org/10.1038/s41598-018-27250-2 (2018).
Zhao, G. et al. HMGB1 mediates the development of tendinopathy due to mechanical overloading. PloS One. 14, e0222369. https://doi.org/10.1371/journal.pone.0222369 (2019).
Zhang, J. et al. Effect of Metformin on development of tendinopathy due to mechanical overloading in an animal model. Foot Ankle Int. 41, 1455–1465. https://doi.org/10.1177/1071100720966318 (2020).
Tang, D. et al. High mobility group box 1 (HMGB1) phenotypic role revealed with stress. Mol. Med. 20, 359–362. https://doi.org/10.2119/molmed.2014.00063 (2014).
Horiuchi, T. et al. Metformin directly binds the alarmin HMGB1 and inhibits its Proinflammatory activity. J. Biol. Chem. 292, 8436–8446. https://doi.org/10.1074/jbc.M116.769380 (2017).
Bai, B., Chen, H. & Metformin A novel weapon against inflammation. Front. Pharmacol. 12, 622262. https://doi.org/10.3389/fphar.2021.622262 (2021).
Saisho, Y. Metformin and inflammation: its potential beyond Glucose-lowering effect. Endocr. Metab. Immune Disord. Drug Targets. 15, 196–205. https://doi.org/10.2174/1871530315666150316124019 (2015).
Piantadosi, C. A. & Suliman, H. B. Transcriptional control of mitochondrial biogenesis and its interface with inflammatory processes. Biochim. Biophys. Acta. 1820, 532–541. https://doi.org/10.1016/j.bbagen.2012.01.003 (2012).
Scheen, A. J. & Paquot, N. Metformin revisited: a critical review of the benefit-risk balance in at-risk patients with type 2 diabetes. Diabetes Metab. 39, 179–190. https://doi.org/10.1016/j.diabet.2013.02.006 (2013).
Dees, C., Chakraborty, D. & Distler, J. H. W. Cellular and molecular mechanisms in fibrosis. Exp. Dermatol. 30, 121–131. https://doi.org/10.1111/exd.14193 (2021).
Cieslik, K. A. et al. AICAR-dependent AMPK activation improves Scar formation in the aged heart in a murine model of reperfused myocardial infarction. J. Mol. Cell. Cardiol. 63, 26–36. https://doi.org/10.1016/j.yjmcc.2013.07.005 (2013).
Peters, J. A., Zwerver, J., Diercks, R. L. & Elferink-Gemser, M. T. Van Den Akker-Scheek, I. Preventive interventions for tendinopathy: A systematic review. J. Sci. Med. Sport. 19, 205–211. https://doi.org/10.1016/j.jsams.2015.03.008 (2016).
Fredberg, U., Bolvig, L. & Andersen, N. T. Prophylactic training in asymptomatic soccer players with ultrasonographic abnormalities in Achilles and patellar tendons: the Danish super league study. Am. J. Sports Med. 36, 451–460. https://doi.org/10.1177/0363546507310073 (2008).
Pope, R., Herbert, R. & Kirwan, J. Effects of ankle dorsiflexion range and pre-exercise calf muscle stretching on injury risk in army recruits. Aust J. Physiother. 44, 165–172. https://doi.org/10.1016/s0004-9514(14)60376-7 (1998).
Murtaugh, B. & Ihm, J. M. Eccentric training for the treatment of tendinopathies. Curr. Sports Med. Rep. 12, 175–182. https://doi.org/10.1249/JSR.0b013e3182933761 (2013).
LaStayo, P. C. et al. Eccentric muscle contractions: their contribution to injury, prevention, rehabilitation, and sport. J. Orthop. Sports Phys. Ther. 33, 557–571. https://doi.org/10.2519/jospt.2003.33.10.557 (2003).
Lewis, T. & Cook, J. Fluoroquinolones and tendinopathy: a guide for athletes and sports clinicians and a systematic review of the literature. J. Athl Train. 49, 422–427. https://doi.org/10.4085/1062-6050-49.2.09 (2014).
Fusini, F. et al. Nutraceutical supplement in the management of tendinopathies: a systematic review. Muscles Ligaments Tendons J. 6, 48–57. https://doi.org/10.11138/mltj/2016.6.1.048 (2016).
Jiang, D., Gao, P., Lin, H. & Geng, H. Curcumin improves tendon healing in rats: a histological, biochemical, and functional evaluation. Connect. Tissue Res. 57, 20–27. https://doi.org/10.3109/03008207.2015.1087517 (2016).
Gulec, A. et al. Effect of Curcumin on tendon healing: an experimental study in a rat model of Achilles tendon injury. Int. Orthop. 42, 1905–1910. https://doi.org/10.1007/s00264-018-4017-5 (2018).
Zhang, J., Brown, R., Hogan, M. V., Onishi, K. & Wang, J. H. Metformin improves tendon degeneration by blocking translocation of HMGB1 and suppressing tendon inflammation and senescence in aging mice. J. Orthop. Research: Official Publication Orthop. Res. Soc. 41, 1162–1176. https://doi.org/10.1002/jor.25470 (2023).
Zhang, J., Brown, R., Hogan, M. V. & Wang, J. H. Mitigating Scar tissue formation in tendon injuries: targeting HMGB1, AMPK activation, and myofibroblast migration all at once. Pharmaceuticals 16 https://doi.org/10.3390/ph16121739 (2023).
Nasri, H., Rafieian-Kopaei, M. & Metformin Current knowledge. J. Res. Med. Sci. 19, 658–664 (2014).
Scheen, A. J. Metformin and lactic acidosis. Acta Clin. Belg. 66, 329–331. https://doi.org/10.2143/ACB.66.5.2062583 (2011).
Karve, T. et al. Long-acting transdermal drug delivery formulations: current developments and innovative pharmaceutical approaches. Adv. Drug Deliv Rev. 210, 115326. https://doi.org/10.1016/j.addr.2024.115326 (2024).
Millar, N. L. et al. Inflammation is present in early human tendinopathy. Am. J. Sports Med. 38, 2085–2091. https://doi.org/10.1177/0363546510372613 (2010).
Mosca, M. J. et al. Differential expression of alarmins-S100A9, IL-33, HMGB1 and HIF-1alpha in supraspinatus tendinopathy before and after treatment. BMJ Open. Sport Exerc. Med. 3, e000225. https://doi.org/10.1136/bmjsem-2017-000225 (2017).
Magna, M. & Pisetsky, D. S. The role of HMGB1 in the pathogenesis of inflammatory and autoimmune diseases. Mol. Med. 20, 138–146. https://doi.org/10.2119/molmed.2013.00164 (2014).
Yuan, J. et al. HMGB1 as an extracellular pro-inflammatory cytokine: implications for drug-induced organic damage. Cell. Biol. Toxicol. 40, 55. https://doi.org/10.1007/s10565-024-09893-2 (2024).
Katzel, E. B. et al. The impact of Smad3 loss of function on TGF-beta signaling and radiation-induced capsular contracture. Plast. Reconstr. Surg. 127, 2263–2269. https://doi.org/10.1097/PRS.0b013e3182131bea (2011).
Li, Y. et al. Transforming growth factor-beta signalling pathway in tendon healing. Growth Factors. 40, 98–107. https://doi.org/10.1080/08977194.2022.2082294 (2022).
Shinde, A. V., Humeres, C. & Frangogiannis, N. G. The role of alpha-smooth muscle actin in fibroblast-mediated matrix contraction and remodeling. Biochim. Biophys. Acta Mol. Basis Dis. 1863, 298–309. https://doi.org/10.1016/j.bbadis.2016.11.006 (2017).
Kahn, B. B., Alquier, T., Carling, D. & Hardie, D. G. AMP-activated protein kinase: ancient energy gauge provides clues to modern Understanding of metabolism. Cell. Metab. 1, 15–25. https://doi.org/10.1016/j.cmet.2004.12.003 (2005).
Oliveira, N. R. et al. Treadmill training increases SIRT-1 and PGC-1 alpha protein levels and AMPK phosphorylation in quadriceps of middle-aged rats in an intensity-dependent manner. Mediat. Inflamm. 987017 https://doi.org/10.1155/2014/987017 (2014).
Spaulding, H. R. & Yan, Z. AMPK and the adaptation to exercise. Annu. Rev. Physiol. 84, 209–227. https://doi.org/10.1146/annurev-physiol-060721-095517 (2022).
Wang, Y. et al. Metformin improves mitochondrial respiratory activity through activation of AMPK. Cell Rep. 29, 1511–1523 e1515 https://doi.org/10.1016/j.celrep.2019.09.070 (2019).
Izzo, A. et al. Metformin restores the mitochondrial network and reverses mitochondrial dysfunction in down syndrome cells. Hum. Mol. Genet. 26, 1056–1069. https://doi.org/10.1093/hmg/ddx016 (2017).
Hold, L. et al. AMPKα1 is necessary for extracellular matrix homeostasis in mouse Achilles tendon. Physiology 38 https://doi.org/10.1152/physiol.2023.38.S1.5730074 (2023).
Maloney, D. J., Zhang, J., Bhargava, S., Hogan, M. V. & Wang, J. H. Quantifying skin permeation of a novel Metformin lotion using modified hydrophilic interaction liquid chromatography. Bioanalysis 16, 1115–1124. https://doi.org/10.1080/17576180.2024.2412438 (2024).
Acknowledgements
The authors thank Bhavani P. Thampatty for her assistance in manuscript preparation.
Funding
This work was supported in part by a Department of Defense/Medical Technology Enterprise Consortium (DOD/MTEC) Award (W81XWH2290016), DOD Award (HT9425-23-1-0617), and Pittsburgh Foundation Awards (AD2021-120112, AD2022-130408, AD2023-134256).
Author information
Authors and Affiliations
Contributions
J.Z.: Contributed Investigation, Data curation, Formal analysis, Validation, Writing—original draft; D.M.: Contributed Methodology, Investigation; V.P.: Contributed Methodology, Investigation, Validation, S.H.:Contributed Methodology, Investigation, Validation, M.V.H.: Contributed Writing—review and editing, J.H-C.: Contributed Conceptualization, Project administration, Methodology, Validation, Resources, Writing—review and editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
The protocol for animal use was approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Pittsburgh (protocol #22091904). The study is reported in accordance with ARRIVE guidelines.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhang, J., Maloney, D., Pastukh, V. et al. Metformin lotion as a novel approach to prevent tendinopathy induced by mechanical overuse. Sci Rep 15, 31474 (2025). https://doi.org/10.1038/s41598-025-16279-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-16279-9