Abstract
Hepatic ischemia‒reperfusion injury (HIRI) is a common pathological phenomenon after hepatectomy and liver transplantation. Here, we aim to explore the role of Axin formation inhibitor 1 (Axin1) in HIRI. In this work, we find that the expression of Axin1 is upregulated after HIRI. Cellular experiments confirme that Axin1 knockdown alleviated hypoxia/reoxygenation (H/R)-induced inflammation and apoptosis. Subsequently, we construct a HIRI model based on transgenic hepatocellular-specific Axin1 knockout and overexpression male mice and find that Axin1 deletion alleviated inflammation and apoptosis. Transcriptome sequencing reveal that the genes whose expression differed after Axin1 overexpression are significantly enriched in the PPAR signaling pathway. Furthermore, we demonstrate that Axin1 negatively regulates the expression of PPARβ, thereby activating the NF-κB pathway. Mechanistically, Axin1 binds to PPARβ to enhance the ubiquitination-mediated degradation of PPARβ by the E3 ubiquitin ligase RBBP6. Notably, adenovirus-mediated Axin1 knockdown block I/R damage in mice. Our study results demonstrate that Axin1 exacerbates HIRI by promoting the ubiquitination and degradation of PPARβ, which in turn activates the NF-κB signaling pathway. These results suggest that Axin1 may be a potential therapeutic target for HIRI.
Similar content being viewed by others
Introduction
Hepatic ischemia‒reperfusion injury (HIRI) is a common complication during hepatectomy and liver transplantation and is a high-risk factor for liver dysfunction and distal organ failure1,2. It has been reported that HIRI is closely related to 10% of early acute rejection of liver transplantation, which greatly affects the therapeutic effect of liver transplantation3. The pathogenesis of HIRI is complex, inflammation and apoptosis are the critical involvement during HIRI. In the ischemic stage, a lack of oxygen and metabolic disorders stimulates inflammatory cells to secrete inflammatory cytokines, induces cell damage4. During the reperfusion stage, mononuclear macrophages and neutrophils recruited from the blood aggravate the intrahepatic inflammatory response and cause inflammatory injury5. Moreover, during HIRI, excessive ROS production can also trigger the secretion of proinflammatory mediators, including cytokines, chemokines, and adhesion molecules, exacerbating apoptosis and tissue damage6. These pathological events mutually promote severe and irreversible liver dysfunction. Although many strategies have been proposed to alleviate HIRI, including ischemic preconditioning and drug therapy, the results are still unsatisfactory7. Therefore, exploration of new therapeutic targets will help to improve the prognosis of HIRI.
Axin formation inhibitor 1 (Axin1) is a multidomain scaffold protein that can play a key regulatory role in different pathophysiological processes, including: inflammation, apoptosis, oxidative stress and so on8. It was identified as an inhibitor of embryonic body axis development9, and has also been shown to inhibit the development and progression of tumors10,11,12,13. Previous studies have confirmed that Axin1 plays an important role in inflammatory response and apoptosis. In myocardial ischemia‒reperfusion injury models, the expression of Axin1 was up-regulation and Axin1 knockdown can inhibit inflammation and apoptosis14,15. In colon cancer, Axin1 can activate the intestinal inflammatory response and promote cell apoptosis16. Axin1 knockdown can also inhibit the expression of IL-6 and TNF-α in alveolar epithelial cells17 and osteoblasts18. Meanwhile, Axin1 can enhance TNF-α induced apoptosis. It has also been found that Axin1 can inhibit the activation of the Wnt signaling pathway, which can inhibit the inflammatory response through the TLR4/NF-κB pathway19. The MAPK pathway is a classic inflammatory signaling pathway, and studies have shown that Axin1 can bind to MEKK1 or MEKK4, thus promoting MEKK phosphorylation and activating enzymatic cascades20. In addition, Axin1 can activate TP53 and PARP signaling pathways, thereby promoting apoptosis21,22. Axin1 has been shown to play a pro-apoptotic role in a variety of cells23. Inflammation and apoptosis play important roles in HIRI, so we speculate that Axin1 may also be involved in the regulation of HIRI.
Peroxisome proliferator-activated receptor β (PPARβ) is a member of the PPAR nuclear hormone receptor family and is widely involved in the regulation of lipid metabolism, glucose homeostasis, and cell differentiation. Studies have shown that PPARβ agonists can promote the oxidative decomposition of fatty acids, thereby inhibiting liver steatosis in mice24,25. PPARβ is also considered a regulator of the inflammatory response26. During myocardial ischemia‒reperfusion injury, PPARβ alleviates cell injury through the AKT and NF-κB signaling pathways27. In the process of renal ischemia‒reperfusion injury, exogenous addition of the PPARβ agonist GW0742 can reduce the inflammatory response and cell injury28. In addition, GW0742 inhibited the proinflammatory effect of NF-κB, thereby alleviating cerebral ischemia‒reperfusion injury in rats29. Our previous study revealed that PPARβ could play an anti-inflammatory and antiapoptotic role in HIRI through the NF-κB signaling pathway30.
In this study, we found that Axin1 expression was upregulated after HIRI. In vitro and in vivo experiments confirmed that Axin1 is involved in the regulation of inflammation and apoptosis in HIRI. In addition, we found that Axin1 negatively regulates the expression of PPARβ, thereby activating the NF-κB signaling pathway and aggravating the inflammatory response and apoptosis in HIRI. Mechanistically, we demonstrated that Axin1 binds to the PPARβ protein and promotes RBBP6-mediated degradation of ubiquitinated PPARβ. The results of this study suggest that Axin1 may be a potential therapeutic target for HIRI.
Results
Hepatic Axin1 expression was upregulated after hepatic I/R injury
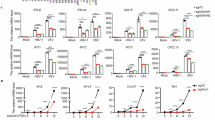
To investigate the role of Axin1 in HIRI, we first determined whether Axin1 expression levels are altered in HIRI patients. The GSE228781 dataset from the GEO database was obtained and analyzed. The results showed that AXIN1 expression was significantly upregulated in the post group after surgery compared to the pre group (Fig. 1A). We also collected normal and ischemia‒reperfusion-subjected liver tissues from the patients (Fig. 1B). The results showed that AXIN1 expression was upregulated after HIRI (Fig. 1C‒E). Subsequently, we analyzed the correlation between the AXIN1 expression level and postoperative liver function of patients, and found that AXIN1 expression was positively correlated with the ALT and AST levels (Fig. 1F, G). To confirm the expression of Axin1 after HIRI, we constructed a mouse HIRI model (Fig. 1H). The results showed that the expression of Axin1 peaked after 6 h of reperfusion (Fig. 1I). Therefore, we chose 6 h as the reperfusion time point for subsequent experiments. Immunohistochemical and Western blotting results showed that Axin1 expression was significantly upregulated after HIRI (Fig. 1J–L). In addition, we isolated primary mouse hepatocytes and constructed a hepatocyte H/R model (Fig. 1M), and the results showed that Axin1 expression was upregulated after H/R (Fig. 1N–P). These results indicate that Axin1 expression is upregulated after HIRI.
A The Axin1 expression was obtained based on GEO data set GSE228781, the samples were divided into pre and post groups (n = 10, P = 0.0078). B Schematic diagram of HIRI sample collection in clinical patients. C The mRNA expression of Axin1 in normal liver tissue and HIRI liver tissue (n = 14, P = 0.041917, 0.002460, 0.034057, 0.000448, 0.002185, 0.000204, 0.005617, 0.025864, 0.001310, 0.004123, 0.000314, 0.110014, 0.000050, 0.004120). D, E The protein expression of Axin1 in normal liver tissue and HIRI liver tissue(n = 6, P = 0.001053,0.011376, 0.013160, 0.010583, 0.049050, 0.039689). F Correlation analysis of the mRNA expression of Axin1 and ALT on the first day after surgery (n = 14). G Correlation analysis of the mRNA expression of Axin1 and AST on the first day after surgery (n = 14). H Schematic diagram of mice HIRI model. I The mRNA expression of Axin1 at different reperfusion time points (n = 5, P = 0.0013). J–L The expression of Axin1 was detected by immunohistochemistry and Western blot (n = 5, P = 0.0014). M Schematic diagram of Primary hepatocyte H/R model. N–P The expression of Axin1 was detected by qRT-PCR and Western blot (n = 3, P = 0.0008, 0.0014). IR ischemia reperfusion, H/R hypoxia/reoxygenation, ALT alaninea minotransferase, AST aspartate aminotransferase. The statistical significance of differences were assessed by Pearson correlation coefficients for (F, G), Other assays were assessed by Two-tailed t-test. The data are presented as mean ± SD. Three biologically independent experiments. Source data are provided as a Source Data file. B, H, M created with BioRender.com released under a Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International license (https://creativecommons.org/licenses/by-nc-nd/4.0/deed.en).
Axin1 knockdown alleviates H/R-induced hepatocyte damage
We then evaluated the function of Axin1 in hepatocytes in response to H/R challenge. Axin1 expression was knocked down in hepatocytes by adenoviral vector transfection (Fig. 2A, B) and the H/R model was constructed (Fig. 2C). CCK-8 and LDH assay results showed that Axin1 knockdown alleviated H/R-induced cell damage (Fig. 2D, E). Apoptosis is an important form of cell death after HIRI, and Bax, Bcl2, and caspase-3 are the key molecules involved in apoptosis. We determined that H/R induced an increase in caspase-3 activity, while Axin1 knockdown inhibited the activity of caspase-3 (Fig. 2F). The qRT‒PCR results showed that Axin1 knockdown inhibited Bax expression. Expression of Bcl2, an antiapoptotic protein, was significantly downregulated after H/R, while Axin1 knockdown restored the expression of Bcl2 (Fig. 2G, H). Similarly, Western blotting also revealed consistent results (Fig. 2I–L). Subsequently, we detected the proportion of apoptotic cells by immunofluorescence and flow cytometry, and the results showed that H/R caused a significant increase in the proportion of apoptotic cells, while the number of apoptotic cells decreased after Axin1 knockdown (Fig. 2M, P). Furthermore, we verified these findings in the human normal hepatocyte cell line WRL68, and the results showed that Axin1 knockdown could alleviate H/R-induced cell damage (Fig S1).
A, B Western blot detects Axin1 knock-down efficiency (n = 3, P = 0.0004). C Diagram of Axin1 knockdown hepatocytes H/R model. D Cell viability was measured by CCK-8 (n = 3, P = 0.0407). E LDH detects cell damage (n = 3, P = 0.0087). F Caspase 3 activity in each group (n = 3, P = 0.0178). G, H The expression of Bax and Bcl2 was detected by qRT-PCR (n = 3, P = 0.0384, 0.0218). I–L The expression of Bax, Bcl2, and c-Caspase3 was detected by Western blot (n = 3, P = 0.0044, 0.0474, 0.0015). M, N The apoptotic cells were detected by immunofluorescence (n = 3, P = 0.0083). O, P The apoptotic cells were detected by flow cytometry (n = 3, P = 0.0113). Q–V qRT-PCR and ELISA detected the expression of inflammatory factors (n = 3, P = 0.018608, 0.001316, 0.048484, 0.0080, 0.0419, 0.0102). WT wild-type, H/R hypoxia/reoxygenation, LDH lactate dehydrogenase, PI propidium iodide, FITC fluorescein isothiocyanate.Two-tailed t-test. The data are presented as mean ± SD. Three biologically independent experiments. Source data are provided as a Source Data file. C created with BioRender.com released under a Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International license (https://creativecommons.org/licenses/by-nc-nd/4.0/deed.en).
The inflammatory response is another core mechanism in the HIRI process, so we examined the expression levels of related inflammatory factors. The qRT‒PCR and ELISA results showed that H/R increased the expression of the proinflammatory cytokines IL-1β, IL-6, and TNF-α, while Axin1 knockdown inhibited the expression of these cytokines (Fig. 2Q‒V). These results indicate that Axin1 knockdown can inhibit the inflammatory response and apoptosis during H/R, thereby reducing cell damage.
Axin1 overexpression exacerbates H/R-induced hepatocyte damage
We next constructed Axin1-overexpression adenoviral vectors and H/R models (Fig. 3A, B). CCK-8 and LDH assays showed that overexpression of Axin1 exacerbated H/R-induced cell damage (Fig. 3C, D). Caspase-3 enzyme activity assays showed that Axin1 overexpression exacerbated H/R-induced caspase-3 enzyme activity (Fig. 3E). The results showed that Axin1 overexpression increased the expression of Bax and cleaved caspase-3 and inhibited the expression of Bcl2 compared with those in the Vector-H/R group (Fig. 3F‒K). Flow cytometry and immunofluorescence results showed that Axin1 overexpression increased the proportion of apoptotic cells induced by H/R (Fig. 3L–O). Moreover, in a WRL68 cell H/R model, we confirmed that Axin1 overexpression aggravated H/R-induced cell damage (Fig S2). In addition, compared with that in the Vector-H/R group, Axin1 overexpression exacerbated the expression of proinflammatory cytokines (Fig. 3P–Q). These results indicate that overexpression of Axin1 can promote inflammation and apoptosis during H/R and aggravate cell damage.
A Western blot detects Axin1 expression (n = 3). B Diagram of Axin1 overexpressed hepatocytes H/R model. C Cell viability was measured by CCK-8 (n = 3, P = 0.0175). D LDH detects cell damage (n = 3, P = 0.0159). E Caspase 3 activity in each group (n = 3, P = 0.0051). F, G The expression of Bax and Bcl2 was detected by qRT-PCR (n = 3, P = 0.027936, 0.017727). H–K The expression of Bax, Bcl2 and c-Caspase3 was detected by Western blot (n = 3, P = 0.027109, 0.000212, 0.000749). L, M The apoptotic cells were detected by immunofluorescence (n = 3, P = 0.0199). N, O The apoptotic cells were detected by flow cytometry (n = 3, P = 0.0235). P, Q qRT-PCR and ELISA detected the expression of inflammatory factors (n = 3, P = 0.029657, 0.006781, 0.031099, 0.0087, 0.0323, 0.0116). WT wild-type, H/R hypoxia/reoxygenation, LDH lactate dehydrogenase, PI propidium iodide, FITC fluorescein isothiocyanate. Two-tailed t-test. The data are presented as mean ± SD. Three biologically independent experiments. Source data are provided as a Source Data file. B created with BioRender.com released under a Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International license (https://creativecommons.org/licenses/by-nc-nd/4.0/deed.en).
Hepatocyte-specific Axin1 expression deficiency ameliorates hepatic I/R injury
Given the potent function of Axin1 expression in hepatocytes, we next examined the role of Axin1 in hepatic I/R injury in vivo. We obtained Axin1-LoxP mice using CRISPR/Cas9 technology (Fig. 4A, Fig. S3) and bred Axin1-LoxP mice with Alb-Cre tool mice to obtain hepatocyte-specific Axin1 knockdown (Axin1-HKO) mice (Fig. 4B). Then, we constructed an Axin1-HKO mouse HIRI model. H&E staining analysis revealed less necrosis in the livers of Axin1-HKO mice than in those of flox mice (Fig. 4C, D). Compared with floxed mice, the serum ALT, AST, and LDH levels were significantly greater in Axin1-HKO mice (Fig. 4E–G).
A Construction of Liver -specific Axin1 knockout mice. B The efficiency of Axin1 knockdown was measured by Western blot (n = 6). C, D H& E staining was used to detect liver tissue injury in mice (n = 6, P = 0.0001). E, F ALT and AST expression level in serum of mice in each group (n = 6, P = 0.0006, <0.0001). G LDH expression level in serum of mice in each group (n = 6, P = 0.0352). H–K The expression of apoptosis-related proteins was detected by Western blot (n = 6, P = 0.000888, 0.020666, 0.004120). L, M Apoptotic cells were detected by Tunel staining (n = 6, P = 0.0001). N, O CD11b positive cells were detected by immunofluorescence staining (n = 6, P = 0.0003). P, Q Ly6G positive cells were detected by immunofluorescence staining (n = 6, P = 0.0005). R The expression of inflammatory factors was detected by qRT-PCR (n = 6, P = 0.001686, 0.000398, 0.009210). HKO hepatocyte knockdown, IR ischemia reperfusion, LDH lactate dehydrogenase, ALT alaninea minotransferase, AST aspartate aminotransferase. Two-tailed t-test. The data are presented as mean ± SD. Three biologically independent experiments. Source data are provided as a Source Data file.
Considering the proapoptotic and proinflammatory effects of Axin1 in hepatocytes, we then validated these results in vivo. Consistently, Axin1-HKO mice exhibited decreased protein expression levels of Bax and cleaved caspase-3 but increased expression levels of Bcl2 (Fig. 4H–K). TUNEL staining showed that Axin1 knockout significantly reduced the number of HIRI-induced apoptotic cells (Fig. 4L–M). Macrophages and neutrophils are the main participants in inflammation in the liver. We determined the expression of CD11b and Ly6G in the HIRI model by immunofluorescence. The results showed that Axin1 knockout inhibited the increase in the number of CD11b-positive cells and Ly6G-positive cells caused by HIRI (Fig. 4N–Q). Subsequently, we determined the expression levels of inflammatory factors by qRT‒PCR and ELISA, and the results showed that Axin1 knockout inhibited the HIRI-induced increase in proinflammatory cytokine levels (Fig. 4R, Fig. S4). The above results confirm that Axin1 knockout can attenuated the inflammatory response and apoptosis and ameliorate the cell damage caused by HIRI.
Hepatocyte-specific Axin1 overexpression aggravates hepatic I/R injury
Subsequently, we further verified in the hepatocyte-specific Axin1 transgenic (Axin1-HTG) mouse HIRI model. We inserted an Axin1 gene fragment with the Alb promoter to obtain Axin1-HTG mice via CRISPR/Cas9 technology (Fig. 5A, Fig. S5). Western blotting analysis revealed that Axin1 expression was significantly increased in the liver tissues of Axin1-HTG mice (Fig. 5B, C). We constructed an Axin1-HTG HIRI mouse model to confirm the function of Axin1 in IR injury. H&E staining analysis revealed larger areas of necrosis in the livers of Axin1-HTG mice than in non-transgenic (NTG) mice (Fig. 5D, E). Compared with NTG mice, the serum ALT, AST, and LDH levels were significantly greater in Axin1-HTG mice (Fig. 5F–H).
A Construction of Liver-specific Axin1 overexpression mice. B, C The expression of Axin1 was measured by Western blot (n = 6, P < 0.0001). D, E H& E staining was used to detect liver tissue injury in mice (n = 6, P = 0.0009). F, G ALT and AST expression level in serum of mice in each group (n = 6, P = 0.0051, 0.0010). H LDH expression level in serum of mice in each group (n = 6, P = 0.0041). I–L The expression of apoptosis-related proteins was detected by Western blot (n = 6, P = 0.023317, 0.029304, 0.001367). M, N Apoptotic cells were detected by Tunel staining (n = 6, P = 0.0002). O, P CD11b positive cells were detected by immunofluorescence staining (n = 6, P = 0.0006). Q, R Ly6G positive cells were detected by immunofluorescence staining (n = 6, P = 0.0001). S The expression of inflammatory factors was detected by qRT-PCR (n = 6, P = 0.000192, 0.007524, 0.000986). HTG hepatocyte transgenic, IR ischemia reperfusion, LDH lactate dehydrogenase, ALT alaninea minotransferase, AST aspartate aminotransferase. Two-tailed t-test. The data are presented as mean ± SD. Three biologically independent experiments. Source data are provided as a Source Data file.
We next examined apoptosis and inflammation. Compared with those in the NTG-IR group, the expression of the Bax and cleaved caspase-3 proteins was increased, and the expression of the Bcl2 protein was decreased in the liver tissue of Axin1-HTG mice (Fig. 5I–L). TUNEL staining revealed that Axin1 overexpression significantly increased the number of TUNEL-positive cells (Fig. 5M, N). Immunofluorescence results showed that overexpression of Axin1 increased the number of CD11b-positive cells and Ly6G-positive cells (Fig. 5O–R). Subsequently, we determined the expression of inflammatory factors by qRT‒PCR and ELISA, and the results showed that overexpression of Axin1 upregulated the expression of IL‒1β, IL‒6 and TNF‒α (Fig. 5S, Fig. S6). Combined with the results of in vivo and in vitro experiments, we confirmed that Axin1 plays a proinflammatory and proapoptotic role in HIRI and exacerbates HIRI-induced cell injury.
Axin1 negatively regulates the expression of PPARβ
To decipher the underlying mechanism of HIRI driven by Axin1 expression upregulation, we performed RNA Sequencing (Fig. 6A). GSEA revealed that Axin1 overexpression significantly activated inflammation and apoptosis signaling pathways, and several related genes had significantly upregulated expression in the AdAxin1-H/R group (Fig. 6B–E). KEGG analysis revealed that the differentially expressed genes (DEG) were significantly enriched in PPAR, P53, IL-17, and other signaling pathways (Fig. 6F). Subsequently, we obtained liver tissue RNA sequencing data from hepatocyte-specific Axin1 knockout mice from the GEO database (GSE107374). KEGG analysis revealed that the DEGs were also enriched in the PPAR signaling pathway (Fig. 6G). Therefore, we speculate that Axin1 may affect the PPAR signaling pathway and thus participate in HIRI regulation. The PPAR signaling pathway is mediated by PPARα, PPARβ, and PPARγ. Therefore, we determined the expression of PPARα, PPARβ, and PPARγ in the AdVector-H/R and AdAxin1-H/R groups. The qRT‒PCR results showed that Axin1 overexpression did not affect the mRNA expression of PPARα, PPARβ, or PPARγ (Fig. S7A‒C). Western blotting results showed that overexpression of Axin1 significantly decreased the expression of PPARβ (Fig. 6H, I), while knockdown of Axin1 upregulated PPARβ expression (Fig. S7D).
A Schematic of RNA sequencing workflow. B Enrichment plot corresponding to Inflammatory response geneset of GSEA. C Heat map of genes commonly associated with inflammation. D Enrichment plot corresponding to Apoptosis geneset of GSEA. E Heat map of genes commonly associated with apoptosis. F KEGG analysis of the differential genes. G Based on the GSE107374 dataset, liver tissue RNA sequencing data of liver-specific Axin1 knockout mice were obtained, KEGG analysis of the differential genes. H–I Expression of PPARα, PPARβ, and PPARγ were detected by western blot (n = 3, P = 0.0002). J–L Expression of P65 and IKBα in cell were detected by western blot (n = 3, P = 0.0054, 0.0052). M–O Expression of P65 and IKBα in mice were detected by western blot (n = 6, P = 0.0245, <0.0001). P, Q The activity of NF-κb (n = 3, P = 0.0009, 0.0103). H/R hypoxia/reoxygenation, HTG hepatocyte transgenic. Two-tailed t-test. The data are presented as mean ± SD. Three biologically independent experiments. Source data are provided as a Source Data file. A created with BioRender. com released under a Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International license (https://creativecommons.org/licenses/by-nc-nd/4.0/deed.en).
We have previously demonstrated that PPARβ can inhibit the NF-κB signaling pathway in HIRI30; therefore, we examined whether Axin1 is also involved in the regulation of NF-κB signaling. In vitro experiments showed that overexpression of Axin1 upregulated the expression of p-P65 and P-IKKα, while knockdown of Axin1 had the opposite effect (Fig. 6J–L, Fig. S8A–C). Furthermore, we obtained the same results in Axin1-HTG and Axin1-HKO mice (Fig. 6M–O, Fig. S8D–F). The NF-κB signaling pathway promotes the transcription of downstream inflammatory molecules, so we examined NF-κB-mediated transcriptional activity. The results showed that overexpression of Axin1 increased NF-κB activity, while knockdown of Axin1 expression inhibited H/R-induced NF-κB activity (Fig. 6P–Q).
PPARβ overexpression alleviates the proinflammatory and proapoptotic effects of Axin1 in HIRI
To verify whether PPARβ mediates the function of Axin1 in HIRI, we performed a rescue experiment. As previously reported30, we transfected PPARβ-overexpressing adenovirus into primary hepatocytes from Axin1-HTG mice and constructed an H/R cell model (Fig. 7A). CCK-8, LDH, and caspase-3 activity assays showed that overexpression of PPARβ reversed the Axin1-induced cell injury (Fig. 7B–D). PPARβ overexpression inhibited the expression of Bax and cleaved caspase-3 and promoted the expression of Bcl2 compared with those in the control group (Fig. 7E–G). Flow cytometry showed that overexpression of PPARβ reduced cell apoptosis (Fig. 7H). Western blotting results showed that overexpression of PPARβ reversed the changes in the expression of p-P65 and p-IKB-α (Fig. 7I, J).
A Schematic diagram of cell H/R model used in the rescue experiment. B Cell viability was measured by CCK-8 (n = 3, P = 0.0012). C LDH detects cell damage (n = 3, P = 0.0380). D Caspase 3 activity in each group (n = 3, P = 0.0293). E, F The expression of Bax and Bcl2 was detected by qRT-PCR (n = 3, P = 0.000819, 0.000392). G The expression of Bax, Bcl2, and c-Caspase3 was detected by Western blot (n = 3). H The apoptotic cells were detected by flow cytometry (n = 3). I Expression of P65 and IKBα in cell were detected by western blot (n = 3). J The activity of NF-κb (n = 3, P = 0.0043). K Schematic diagram of mice HIRI model used in the rescue experiment. L, M H& E staining was used to detect liver tissue injury in mice (n = 6, P = 0.0011). N, O ALT and AST expression level in serum of mice in each group (n = 6, P = 0.0018, 0.0003). P The expression of apoptosis-related proteins was detected by Western blot (n = 6). Q Expression of P65 and IKBα in cell were detected by western blot (n = 6). H/R hypoxia/reoxygenation, HTG hepatocyte transgenic, LDH lactate dehydrogenase, PI propidium iodide, FITC fluorescein isothiocyanate. Two-tailed t-test. The data are presented as mean ± SD. Three biologically independent experiments. Source data are provided as a Source Data file. A, K created with BioRender. com released under a Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International license (https://creativecommons.org/licenses/by-nc-nd/4.0/deed.en).
Subsequently, in NTG and Axin1-HTG mice, we injected a PPARβ overexpressing adenoviral vector via the tail vein and constructed a mouse HIRI model (Fig. 7K and Fig. S7E). H&E staining revealed that compared with NTG mice, PPARβ overexpression reduced the necrotic area (Fig. 7L–M). PPARβ overexpression downregulated ALT and AST levels (Fig. 7N–O). Western blotting analysis revealed that overexpression of PPARβ decreased the expression of the proapoptotic proteins Bax and cleaved caspase-3, increased the expression of the antiapoptotic protein Bcl2 (Fig. 7P), and reversed the Axin1-induced upregulation of p-P65 and p-IKBα expression (Fig. 7Q). The above experiments demonstrated that Axin1 promoted inflammation and apoptosis by inhibiting the expression of PPARβ and aggravated HIRI.
Axin1 promotes the ubiquitination and degradation of PPARβ
In the above experiments, we found that the overexpression of Axin1 inhibited PPARβ protein expression (Fig. 6H) but did not affect PPARβ mRNA expression (Fig. S7B). This finding suggests that Axin1 may be involved in the posttranscriptional regulation of PPARβ. CHX experiments confirmed that overexpression of Axin1 promoted the degradation of the PPARβ protein (Fig. 8A, B), while knockdown of Axin1 expression inhibited the degradation of the PPARβ protein (Fig. S9A, B). Subsequently, the protease inhibitor MG132 and lysosomal inhibitors chloroquine (CQ) were added to block the ubiquitin‒proteasome and autophagolysosomal pathways, respectively. MG132 inhibited the PPARβ protein degradation induced by Axin1 (Fig. 8C, D). These results suggest that Axin1 may promote the ubiquitination and degradation of PPARβ. To verify this hypothesis, we overexpressed Axin1 in hepatocytes and measured PPARβ ubiquitination levels. The results showed that overexpression of Axin1 significantly increased PPARβ polyubiquitination (Fig. 8E), while knockdown of Axin1 expression had the opposite effect (Fig. S9C). Subsequently, we cotransfected HEK-293T cells with Axin1, PPARβ, and Ub plasmids, and the results showed that Axin1 significantly increased the ubiquitination of PPARβ (Fig. 8F).
A, B The effect of overexpression of Axin1 on the half-lives of PPARβ in hepatocyte treated with CHX (n = 3, P = 0.0112, 0.0029). C, D The expression of PPARβ is detected by Western blot after treating with MG132 or CQ (n = 3, P = 0.000220, 0.071143, 0.000757). E Co-IP shows the ubiquitination level of PPARβ in hepatocyte treated with MG132 (n = 3). F Co-IP shows the ubiquitination level of PPARβ in HEK-293T treated with MG132 (n = 3). G Axin1 colocalized with PPARβ in the cytoplasm of hepatocyte (n = 3). H Molecular docking was employed to predict the binding interactions between Axin1 and PPARβ. I, J The immunoprecipitation of Axin1 and PPARβ was detected in hepatocyte (n = 3). K Full-length of Axin1 and its truncated forms (D6) with PPARβ were co-transfected into HEK-293T cells, IP with anti-Flag antibody followed by western blot (n = 3). L Full-length of PPARβ and its truncated forms (D1, D2, D3) with Axin1 were co-transfected into HEK-293T cells, IP with anti-His antibody followed by western blot (n = 3). M Full-length of Axin1 and its truncated forms (D6) with PPARβ were co-transfected into HEK-293T cells, Co-IP shows the ubiquitination level of PPARβ in HEK-293T treated with MG132 (n = 3). N V Secondary protein structure diagram of PPARβ. O The immunoprecipitation of Axin1, PPARβ, and RBBP6 was detected in HEK-293T (n = 3). P Co-transfected Axin1, PPARβ and siRBBP6, Co-IP shows the ubiquitination level of PPARβ (n = 3). IP immunoprecipitation, CHX cycloheximide, DMSO Dimethyl Sulfoxide, CQ chloroquine. Two-tailed t-test. The data are presented as mean ± SD. Three biologically independent experiments. Source data are provided as a Source Data file.
Previous studies have shown that Axin1 can bind to a variety of proteins, thereby promoting their ubiquitination-mediated degradation31. Therefore, we speculated that Axin1 can bind to the PPARβ protein. Immunofluorescence staining revealed that Axin1 and PPARβ were colocalized in the cytoplasm (Fig. 8G and Fig. S10A). Molecular docking analysis results indicated that Axin1 strongly bound to PPARβ (Fig. 8H). We then overexpressed Axin1 and PPARβ in hepatocytes, and co-IP results showed that Axin1 could bind to PPARβ (Fig. 8I, J). Subsequently, we cotransfected HEK-293T cells with Axin1 and PPARβ plasmids and obtained consistent results (Fig. S10B, C). To explore potential binding regions, we analyzed the results of molecular docking analysis (Fig. S10D) and found that the sites with the strongest binding forces were Axin1 (aa 691 region) and PPARβ (aa127 region). Furthermore, we constructed serially truncated Axin1 and PPARβ constructs and performed co-IP to identify the exact regions of Axin1 and PPARβ that mediate their interaction. Notably, Axin1 (aa507-757 region) and PPARβ (aa73-440 region) mediated the interaction between these two proteins (Fig. 8K–L and Fig. S10E).
Since Axin1 has no ubiquitination function, we hypothesized that Axin1 may affect the binding of the E3 ubiquitin ligase to PPARβ. Therefore, we obtained Axin1-binding proteins by co-IP and mass spectrometry (Fig. S11A). Subsequently, we predicted potential PPARβ E3 ubiquitin ligases using the ubibrowser database (Fig. S11B). By intersecting the Axin1 binding protein with potential PPARβ E3 ubiquitin ligases (Fig. S11C), we found that RBBP6 may mediate the pro-ubiquitination effect of Axin1 on PPARβ (Fig. 8N). Molecular docking and co-IP results showed that Axin1, PPARβ, and RBBP6 bound to one another (Fig. 8O and Fig. S11D). Finally, we inhibited RBBP6 expression in HEK-293T cells and transfected them with Axin1, PPARβ, and Ub plasmids. The results showed that silencing RBBP6 expression significantly inhibited Axin1-mediated PPARβ ubiquitination (Fig. 8P). K48 polyubiquitination of proteins is mainly involved in protein degradation, we transferred to wild type and K48 mutant Ub plasmids, and found that K48R reversed the ubiquitination of PPARβ (Fig. S12). The general schematic diagram is shown in Fig. S13.
Axin1 knockdown alleviates HIRI in mice
To explore the potential therapeutic value of Axin1, we injected Axin1 knockdown adenoviral vector via the tail vein and constructed an HIRI model (Fig. S14A). Western blotting results showed that the expression of Axin1 was decreased in mouse liver tissue (Fig. S14B, C). H&E staining revealed that the necrotic area of liver tissues in the Axin1-knockdown group was significantly reduced (Fig. S14D, E). Compared with those in the control group, the levels of ALT, AST, and LDH were lower in the Axin1-knockdown group (Fig. S14F–H). TUNEL staining showed that Axin1 knockdown reduced the number of apoptotic cells induced by HIRI (Fig. S14I, J). In addition, Axin1 knockdown downregulated the expression of Bax and cleaved caspase-3 and promoted the expression of Bcl2 (Fig. S14K–L).
Discussion
HIRI is a complex pathophysiological process that greatly affects the prognosis of patients after liver surgery. Although the mechanism of HIRI and treatment strategies have been extensively studied in the past, there is still a lack of effective treatments in clinical practice. In this study, we found that the expression of Axin1 was upregulated after HIRI in patients and mice. Through gain- and loss-of-function experiments, we found that Axin1 expression exacerbated inflammation and apoptosis during HIRI. Mechanistically, Axin1 binds to PPARβ, thereby enhancing RBBP6-mediated PPARβ ubiquitination-mediated degradation and activating the NF-κB signaling pathway. These results suggest that Axin1 may be an important target for alleviating HIRI.
Axin1 regulates ischemia‒reperfusion injury in various tissues and organs. We confirmed that Axin1 plays a proinflammatory and proapoptotic role during HIRI in vitro and in vivo, which ultimately exacerbates HIRI. Consistent with our results, Jian et al.32 reported that Axin1 expression was increased in HIRI mice. Similarly, in a cardiomyocyte H/R model, Axin1 expression was increased, and Axin1 knockout alleviated apoptosis and the inflammatory response caused by H/R injury14,15. Interestingly, Axin1 expression was upregulated in oxygen/glucose deprivation models of human microvascular endothelial cells and improved cerebral ischemia‒reperfusion injury by upregulating the expression of tight junction proteins33. The above results indicate that Axin1 expression is upregulated under hypoxic reoxygenation stimulation but may play different roles in different types of cells. In addition, Axin1 expression is downregulated in the repair phase of myocardial infarction34, which suggests that Axin1 may participate in the repair process of I/R injury.
Inflammation plays an important role in HIRI and Axin1 is involved in regulating inflammatory responses in a variety of disease models. Its knockdown can inhibit the expression of IL-6 and TNF-α in alveolar epithelial cells17 and osteoblasts induced by LPS18. In a colitis model, Axin1-specific deletion of intestinal epithelial cells downregulated the expression of IL-1β, IL-6, TNF-α, CCL2, and other inflammatory cytokines by inhibiting the NF-κB signaling pathway16. In this study, we found that Axin1 deletion decreased HIRI-induced increases in the expression of IL-1β, IL-6, and TNF-α. XAV939 is a tankyrase inhibitor that inhibits Axin1 degradation. Studies have shown that XAV939 can upregulate the expression of IL-6 and TNF-α in cerebral ischemia‒reperfusion injury35. In addition, we found that Axin1 overexpression promoted HIRI-induced CD11b-positive and Ly6G-positive inflammatory cell infiltration, while hepatocellular-specific Axin1 knockout had the opposite effect. This finding suggests that Axin1 expression in hepatocytes is involved in regulating the inflammatory response during liver I/R injury by influencing the recruitment of inflammatory cells and the expression of inflammatory factors.
Apoptosis is the main form of hepatocyte death after HIRI stimulation21. Axin1 plays an important role in the regulation of apoptotic signals. In 293 cells, Axin1 activated TP53 transcriptional activity by binding to TP53, thereby promoting apoptosis22. Studies have shown that Axin1 can activate PARP and cause the release of mitochondrial apoptosis-inducing factors, which aggravate apoptosis36. Axin1 can also promote the expression of Bax and inhibit the expression of Bcl2 in NTera2 cells through the PI3K/AKT/mTOR signaling pathway and ultimately promote apoptosis37. Zhang et al.38 reported that the overexpression of Axin1 promoted the apoptosis of mouse neurons. In an AC16 cell ischemia‒reperfusion injury model, Axin1 expression promoted cardiomyocyte apoptosis through the SIRT1/NRF2 signaling pathway14. In this study, we confirmed that Axin1 is involved in the regulation of HIRI-induced apoptosis. Compared with the control group, Axin1 knockdown increased the expression of antiapoptotic Bcl2 and decreased the expression of proapoptotic Bax and cleaved caspase-3, while Axin1 overexpression had the opposite effect. These results suggest that Axin1 is an effective regulator of apoptosis during ischemia‒reperfusion injury.
PPARβ plays an important role in the regulation of ischemia‒reperfusion injury. Its agonists can inhibit the expression of proinflammatory cytokines, chemokines, and adhesion molecules26. PPARβ plays an anti-inflammatory and antiapoptotic role in myocardial ischemia‒reperfusion injury27. Our previous studies revealed that PPARβ can inhibit the NF-κB signaling pathway, reduce the expression of inflammatory factors, reduce cell apoptosis, and alleviate HIRI30. In this study, we confirmed that Axin1 can downregulate the expression of PPARβ and thus play a proinflammatory and proapoptotic role. Previous studies have confirmed that the Wnt signaling pathway is one of the main pathways by which Axin1 regulates inflammation and apoptosis. Interestingly, we found that the PPAR signaling pathway, but not the Wnt signaling pathway, mediates the proinflammatory and proapoptotic effects of Axin1 in HIRI. The reason for this result may be related to Axin2, a homologous protein of Axin1. Studies have shown that Axin1 and Axin2 have redundant functions in some diseases38. The transcription of Axin2 can be upregulated by Wnt signaling, and Axin2 expression can negatively regulate Wnt signaling pathways39. A similar study revealed that the Wnt signaling pathway was not activated in a mouse Axin1 knockout liver cancer model10. This finding suggests that the regulatory effect of Axin1 on the Wnt signaling pathway may be offset by the negative feedback regulatory effect of Axin2.
The ubiquitin proteasome pathway is an important method of protein degradation and plays an important role in many cellular activities, such as DNA repair, signal transduction, transcription and translation, and the immune response. In this study, we found that Axin1 (aa507-757 region) binds to PPARβ (aa73-440 region) to enhance the ubiquitination-mediated degradation of PPARβ by the E3 ubiquitin ligase RBBP6. As a multifunctional framework protein, Axin1 can regulate ubiquitination by binding to specific proteins. In the Wnt signaling pathway, Axin1 can act as a scaffold protein to form a complex with β-catenin, APC, GSK3β and CK1α, thereby promoting the degradation of β-catenin and inhibiting the Wnt signaling pathway40. Axin1 knockdown increases the NRF2 protein level via the central region of Axin1 binding to the Neh4/Neh5 domain of NRF2, thus promoting the ubiquitination and degradation of the NRF2 protein31. The E3 ubiquitin ligase RNF146 can form protein complexes with Axin1 and TNKS1 to promote the ubiquitination-mediated degradation of target proteins41. Axin1 can also promote the degradation of Smad7 through the ubiquitin proteasome pathway42, which is dependent on a polymeric complex composed of Axin1, Smad7, and the ubiquitin E3 ligase Arkadia43. Axin1 can also form complexes with the E3 ubiquitin ligases RNF25 and NKD1 to regulate the Wnt signaling pathway44. This finding is consistent with our finding that Axin1, an enhancer of protein ubiquitination, promotes the ubiquitination-mediated degradation of downstream proteins. However, Axin1 can also inhibit the ubiquitination and degradation of downstream target proteins. Axin1 can bind competitively with FANCL and GSK3β, thereby inhibiting the ubiquitination-mediated degradation of FANCL45. This is due to differences in protein binding sites; when Axin1 competes for the same binding site with the E3 ubiquitin ligase of the downstream target protein, Axin1 has been shown to inhibit ubiquitination.
In conclusion, we demonstrate the proinflammatory and proapoptotic effects of Axin1 on HIRI. It exacerbates HIRI by promoting the ubiquitination and degradation of PPARβ, which in turn activates the downstream NF-κB signaling pathway. Our studies provide insight into a potential therapeutic target and useful prognostic biomarker for hepatic I/R injury.
Methods
Ethics approval
All the animal experimental protocols were approved by the Ethics Committee of the First Affiliated Hospital of Harbin Medical University (No.2021087). Informed written consent was obtained from all participants as well. All procedures involving human samples were approved by the Ethics Committee of the First Affiliated Hospital of Harbin Medical University (IRB-AF/SC-04/02.0).
Sex as a biological variable
For clinical human donor liver samples, both sexes were examined. For animal models, the Estrogen has been shown to participate in regulating HIRI, therefore only male mice were examined to reduce female sexual cycle–related variation.
Human liver samples
The human liver tissues used in this study were collected from patients who underwent partial hepatectomy due to benign liver diseases at the First Affiliated Hospital of Harbin Medical University. Normal liver tissue before ischemia and liver tissue samples of ischemia for a total of 30–60 min and reperfusion for 1–2 h were collected. Then the sample was frozen in liquid nitrogen immediately after collection and stored at −80 °C.
Animals
The C57BL/6J mice used in this study were purchased from Beijing Vital River Laboratory Animal Technology Co., Ltd. Male mice aged 6–8 weeks and weighing 18-22 g were used in this study. The mice were divided randomly into the experimental and control groups. All mice were kept in the SPF Laboratory Animal Center of the First Affiliated Hospital of Harbin Medical University with a 12-h light/dark cycle at 20–25 °C with 50–70% humidity, and free access to water and food.
Hepato-specific Axin1 knockout and hepato-specific Axin1 transgenic mice were obtained from Gempharmatech Co., Ltd. The ES cell background was C57BL/6JGpt and the construction strategy of Axin1-HKO mice is as follows: According to the structure of Axin1 gene, exon2-exon9 of Axin1-201 (ENSMUST00000074370.9) transcript is used as the knockout region. The region contains start codon ATG. CRISPR/Cas9 system and Donor were microinjected into the fertilized eggs of C57BL/6JGpt mice. Fertilized eggs were transplanted to obtain positive F0 mice which were confirmed by PCR and sequencing. A stable F1 generation mouse model was obtained by mating positive F0 generation mice with C57BL/6JGpt mice. The flox mice will be knocked out after mating with mice expressing Cre recombinase, resulting in the loss of function of the target gene in specific tissues and cell types.
The construction strategy of Axin1-HTG mice is as follows: Axin1-201 (ENSMUST00000074370.11) was selected for this scheme with a CDS length of 2592 nt and a code of 863 aa. In this project, CRISPR-Cas 9 was used to specifically insert the H11 gene fragment in mouse. The brief procedure is as follows: in vitro vector construction, CRISPR-Cas 9, and Donor vector were microinjected into the fertilized eggs of C57BL/6JGpt mice to obtain F0 generation mice. Correct F0 generation positive mice verified by PCR and sequencing yielded a stably inherited F1 generation positive mice model mated with C57BL/6JGpt mice.
Mouse HIRI model
Mice were anesthetized with 2% pentobarbital sodium (50 mg/kg). The abdominal cavity was exposed along the midabdominal line, and the hepatic hilar vessels were separated. The left lobe and middle lobe of the liver were blocked by a noninvasive arterial clamp, which was removed after 75 min to restore blood supply to the liver. Blood flow blockade was not performed in the Sham group. Liver tissue and serum were collected according to the preset reperfusion time points.
Examination of liver function
The serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST) concentrations were measured using ALT and AST kits from Nanjingjiancheng Company. The procedure was performed according to the manufacturer’s protocols, and the absorbance was determined at 510 nm.
Hematoxylin and eosin (H&E) staining
Liver tissue samples were fixed in 4% paraformaldehyde, dehydrated, and paraffin-embedded. The paraffin-embedded liver tissue was cut into 5 μm thick sections and stained with H&E (Beyotime, China). Images were acquired under an optical microscope.
Terminal dUTP nick-end labeling (TUNEL) assay
Roche TUNEL staining reagent was used for the experiment. The procedure was performed according to the manufacturer’s protocols, and the images were observed and collected under a fluorescence microscope.
Immunofluorescence (IF) and immunohistochemical (IHC) staining
The paraffin sections were subjected to deparaffinization, rehydration, EDTA antigen retrieval, and blocking with 10% BSA at 37 °C for 1 h. Primary antibodies were added to the sections, which were then incubated at 4 °C overnight. Then, the sections were incubated with the corresponding secondary antibodies at 37 °C for 1 h. The images were observed and collected under a fluorescence microscope and light microscope, respectively.
Lactate dehydrogenase (LDH) content determination
The serum LDH concentrations were measured with an LDH kit from Nanjingjiancheng Company. The procedure was performed according to the manufacturer’s protocols, and the absorbance was determined at 450 nm.
RNA isolation and quantitative real-time PCR
According to the manufacturer’s instructions, total RNA was extracted from cells and tissues using a total RNA preparation kit (Axygen, USA), and cDNA was synthesized using a reverse transcription kit (TOYOBO, Japan). qRT‒PCR was performed using SYBR qPCR Mix (Roche, Switzerland) on a real-time PCR system (Applied Biosystems 7500HT Instrument, China). The relative expression levels of genes were calculated by the 2-ΔΔCt method. The sequences of primers used in this study are shown in Supplementary Data 1.
Western blotting
RIPA lysis buffer was used to extract proteins from liver tissues and cells. The protein samples were separated on SDS‒polyacrylamide gels and transferred to PVDF membranes (Merck Millipore Ltd., Germany). The membrane was blocked with 5% skim milk in PBST for 1 h at room temperature and then incubated with primary antibodies overnight at 4 °C. Finally, the membrane was incubated with IRDye 800CW secondary antibodies (LI-COR USA, 925-32210, 1:10000) at room temperature for 1 h, and an Odyssey® Imaging System (LI-COR, USA) was used to visualize and analyze the protein bands. The primary antibodies against the following target proteins were used: Axin1 (Cell Signaling Technology, 2087, 1:1000), PPARα (Proteintech, 66826-1-Ig, 1:1000), PPARβ (Proteintech, 60193-1-Ig, 1:1000), PPARβ (Santa Cruz, sc-74517, 1:1000), PPARγ (Proteintech, 16643-1-AP, 1:1000), RBBP6 (Proteintech, 11882-1-AP, 1:1000), p-IκBα (Cell Signaling Technology, 2859, 1:1000), IκBα (Cell Signaling Technology, 4812, 1:1000), p-P65 (Cell Signaling Technology, 3033, 1:1,000), P65 (Cell Signaling Technology, 4764, 1:1000), Bax (Cell Signaling Technology, 2772, 1:1,000), Bcl-2 (Cell Signaling Technology, 3498, 1:1,000), c-CASP3 (Cell Signaling Technology, 9664, 1:500), FLAG (Proteintech, 66008-4-Ig, 1:1000), His (Proteintech, 66005-1-Ig, 1:1000), HA (Proteintech, 51064-2-AP, 1:1000), Ubiquitin (Santa Cruz, sc-166553, 1:1000), GAPDH (Kangcheng, KC-5G4, 1:8,000).
Isolation of primary hepatocytes
Mice were injected with calcium-free Hank’s solution and IV collagenase solution successively via the portal vein for in situ digestion. The liver tissue was separated, cut, and then added to IV collagenase solution for in vitro digestion. After 70 μm filtration and centrifugation (50 × g, 5 min), hepatocytes were obtained.
Cell hypoxia/reoxygenation (H/R) model
The WRL68 cell line was purchase from Cellverse Co., Ltd. (iCell-h227). Cell medium was replaced with sugar-free and serum-free DMEM, and the cells were placed in a chamber (Biospherix, Lacona, NY, USA) containing 1% O2, 5% CO2, and 94% N2 for 6 h. Then, the medium was replaced with DMEM supplemented with 10% serum, and the cells were incubated under normoxic conditions in 95% air and 5% CO2 for 6 h. The cells were processed for a Cell Counting Kit-8 assay (Servicebio, G4103), LDH assay, caspase-3 activity assay (Beyotime, C1115), and apoptotic cell detection (Beyotime, C1062S).
Enzyme-linked immunosorbent assay (ELISA)
According to the manufacturer’s instructions, the serum and cell culture medium levels of IL-1β, IL-6, and TNF-α were measured with enzyme-linked immunosorbent assay (ELISA) kits (R&D Systems, Minneapolis, MN).
Construction and transfection of adenovirus
The Axin1 knockdown, Axin1 overexpression, PPARβ knockdown, and PPARβ overexpression adenoviral vectors were purchased from Hanbio Biotechnology Co., Ltd. (Shanghai, China). Hepatocytes were counted and seeded into 6-well plates. Adenovirus-containing medium (MOI = 100) was added to the 6-well plates and replaced with normal medium 8 h later. Information on all the target sequences is provided in Supplementary Data 2.
Construction and transfection of plasmids
The full-length homo Axin1 cDNA was cloned into pcDNA3.1-Flag to express Flag-tagged Axin1 recombinant proteins and full-length homo PPARβ cDNA was cloned into pcDNA3.1-His to express His-tagged PPARβ recombinant proteins. Expression plasmids encoding truncated were amplified using PCR and cloned into pcDNA3.1.The plasmids used in this study were purchased from Sangon Biotech (Shanghai) Co., Ltd. The cells were counted and seeded into 6-well plates, and 4 µg of plasmid was transfected into the cells using Lipofectamine 2000.
RNA sequencing and data processing
For RNA-seq analysis, total RNA was extracted from AdVector- and AdAxin1-treated hepatocytes subjected to H/R, after which cDNA libraries were constructed. The libraries were subsequently sequenced using a BGISEQ-500 platform (MGI Tech Co., Ltd., Shenzhen, China). HISAT2 (version 2.21), SAMtools (version 1.4) and StringTie (version 1.3.3b) software were used to analyze the raw data. The gene expression (Supplementary Data 3) and statistical significance were analyzed with DESeq2 software.
Gene set enrichment analysis (GSEA)
Based on the sequencing data, GSEA was performed using the clusterProfiler package (3.14.3) in R software (4.2.1).
KEGG pathway enrichment analysis
Based on sequencing data and the GEO dataset GSE107374, GSE151648, and GSE228781, differentially expressed genes (DEGs) were identified, and Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analysis was performed using the clusterProfiler package (3.14.3) in R software (4.2.1).
CHX assay
Cells were transfected with empty vector or Axin1 adenovirus and cultured with cycloheximide (CHX) (20 μM). Then, the cells were collected at the indicated time points and prepared for Western blotting analysis.
Coimmunoprecipitation and ubiquitination assays
The cells were lysed in IP buffer for 30 min and centrifuged (12,000 × g, 15 min) to prepare a cell protein suspension. The corresponding primary antibody and IgG antibody were added and incubated overnight (4 °C). The next day, agarose beads were added and incubated (4 °C, 3 h). The beads were washed with IP buffer and then centrifuged (12,000 × g, 1 min) to obtain the beads. Protein loading buffer was added, and the samples were boiled (100 °C, 5 min) and then used for Western blotting and mass spectrometry detection. For the ubiquitination assays, cell lysates prepared with IP lysis buffer were used for immunoprecipitation of PPARβ proteins. The level of PPARβ ubiquitination was determined using anti-Ub or anti-HA antibodies.
Molecular docking analysis
The HDOCK online website (http://hdock.phys.hust.edu.cn/) was used for molecular docking analysis. SWISSMODEL was used to model the AXIN1, PPARβ and RBBP6 sequences. Ligplus software was used to analyze the forces between two proteins in two dimensions. PyMOL (version 4.3.0) software was used to map interacting amino acid residues between two proteins.
NF-κB activity assay
An NF-κB plasmid (Beyotime, China), pRL-SV40-N (Beyotime, China) and corresponding adenoviral vector were transfected into hepatocytes, and H/R models were constructed. A dual luciferase reporter gene assay kit (Beyotime, China) was used to detect NF-κB activity according to the manufacturer’s instructions.
Intravenous injection into the mouse tail vein
The Axin1 knockdown adenovirus (1 × 109 pfu per mouse) was injected into mice through the tail vein. After 3 days, the mouse HIRI model was established, and liver tissue and serum were collected.
Statistics and artworks
Statistical analysis was performed with SPSS 19.0 software and GraphPad Prism 9.5 software. The data are presented as the means ± standard deviations (SDs). Student’s t test or one-way or two-way ANOVA was used to compare the differences between independent samples. Correlations were analyzed by Pearson correlation coefficients. P < 0.05 was considered to indicate statistical significance. Artworks in figures were created with BioRender.com (Academic License Terms, www.biorender.com).
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The public RNA-seq used in this study are available in the GSE228781 and GSE107374. The GSE107374 was reported by Abitbol et al.10 and the GSE228781 was reported by Groiss et al.46 in their previous studies. The RNA-seq data generated in this study have deposited in the Genome Sequence Archive (GSA) under accession code CRA022335. All other data of this study are available with in the article and its Supplementary Files. Source data are provided with this paper.
References
Li, F. et al. SIRT1 alleviates hepatic ischemia-reperfusion injury via the miR-182-mediated XBP1/NLRP3 pathway. Mol. Ther. Nucleic Acids 23, 1066–1077 (2021).
Nastos, C. et al. Global consequences of liver ischemia/reperfusion injury. Oxid. Med. Cell. Longev. 2014, 906965 (2014).
Song, D. et al. Tracking hepatic ischemia-reperfusion injury in real time with a reversible NIR-IIb fluorescent redox probe. Angew. Chem. Int. Ed. Engl. 61, e202212721 (2022).
Zhou, J. et al. TNFAIP3 interacting protein 3 is an activator of hippo-YAP signaling protecting against hepatic ischemia/reperfusion injury. Hepatology 74, 2133–2153 (2021).
Kan, C. et al. Ischemia-reperfusion injury in aged livers-the energy metabolism, inflammatory response, and autophagy. Transplantation 102, 368–377 (2018).
Ni, M. et al. Loss of macrophage TSC1 exacerbates sterile inflammatory liver injury through inhibiting the AKT/MST1/NRF2 signaling pathway. Cell Death Dis. 15, 146 (2024).
Gao, W. et al. Nuclear Acly protects the liver from ischemia-reperfusion injury. Hepatology 80, 1087–1103 (2024).
Qiu, L. et al. The scaffold protein AXIN1: gene ontology, signal network, and physiological function. Cell Commun. Signal. 22, 77 (2024).
Shu, B. et al. Inhibition of Axin1 in osteoblast precursor cells leads to defects in postnatal bone growth through suppressing osteoclast formation. Bone Res. 8, 31 (2020).
Abitbol, S. et al. AXIN deficiency in human and mouse hepatocytes induces hepatocellular carcinoma in the absence of β-catenin activation. J. Hepatol. 68, 1203–1213 (2018).
Song, Z. et al. MicroRNA-1181 supports the growth of hepatocellular carcinoma by repressing AXIN1. Biomed. Pharmacother. 119, 109397 (2019).
Zhang, Y. et al. UBE3C promotes proliferation and inhibits apoptosis by activating the β-catenin signaling via degradation of AXIN1 in gastric cancer. Carcinogenesis 42, 285–293 (2021).
Li, T. et al. Starvation induced autophagy promotes the progression of bladder cancer by LDHA mediated metabolic reprogramming. Cancer Cell Int. 21, 597 (2021).
Li, J. et al. Ischemia-reperfusion injury in human AC16 cardiomyocytes is modulated by AXIN1 depending on c-Myc regulation. Ann. Med. Surg. 85, 4844–4850 (2023).
Chen, Q. et al. miR-3574 ameliorates intermittent hypoxia-induced cardiomyocyte injury through inhibiting Axin1. Aging 13, 8068–8077 (2021).
Sanson, R. et al. Axin1 protects colon carcinogenesis by an immune-mediated effect. Cell. Mol. Gastroenterol. Hepatol. 15, 689–715 (2023).
Zhang, Y. et al. Axin-1 binds to Caveolin-1 to regulate the LPS-induced inflammatory response in AT-I cells. Biochem. Biophys. Res. Commun. 513, 261–268 (2019).
Zhang, K. et al. Axin 1 knockdown inhibits osteoblastic apoptosis induced by Porphyromonas gingivalis lipopolysaccharide. Arch. Oral. Biol. 112, 104667 (2020).
Lee, H., Bae, S., Choi, B. W. & Yoon, Y. WNT/β-catenin pathway is modulated in asthma patients and LPS-stimulated RAW264.7 macrophage cell line. Immunopharmacol. Immunotoxicol. 34, 56–65 (2012).
Zhang, Y. et al. Axin forms a complex with MEKK1 and activates c-Jun NH(2)-terminal kinase/stress-activated protein kinase through domains distinct from Wnt signaling. J. Biol. Chem. 274, 35247–35254 (1999).
Wang, H. et al. Role of death receptor, mitochondrial and endoplasmic reticulum pathways in different stages of degenerative human lumbar disc. Apoptosis 16, 990–1003 (2011).
Rui, Y. et al. Axin stimulates p53 functions by activation of HIPK2 kinase through multimeric complex formation. EMBO J. 23, 4583–4594 (2004).
Li, J. et al. Alterations of axis inhibition protein 1 (AXIN1) in hepatitis B virus-related hepatocellular carcinoma and overexpression of AXIN1 induces apoptosis in hepatocellular cancer cells. Oncol. Res. 20, 281–288 (2013).
Regueira, M. et al. Apoptotic germ cells regulate Sertoli cell lipid storage and fatty acid oxidation. Reproduction 156, 515–525 (2018).
Bojic, L. A. et al. PPARδ activation attenuates hepatic steatosis in Ldlr-/- mice by enhanced fat oxidation, reduced lipogenesis, and improved insulin sensitivity. J. Lipid Res. 55, 1254–1266 (2014).
Liu, Y. et al. The role of PPAR-δ in metabolism, inflammation, and cancer: many characters of a critical transcription factor. Int J. Mol. Sci. 19, 3339 (2018).
Kapoor, A. et al. Activation of peroxisome proliferator-activated receptor-beta/delta attenuates myocardial ischemia/reperfusion injury in the rat. Shock 34, 117–124 (2010).
Collino, M. et al. Peroxisome proliferator-activated receptor β/δ agonism protects the kidney against ischemia/reperfusion injury in diabetic rats. Free Radic. Biol. Med. 50, 345–353 (2011).
Kuang, G. et al. Modulation of preactivation of PPAR-β on memory and learning dysfunction and inflammatory response in the hippocampus in rats exposed to global cerebral ischemia/reperfusion. PPAR Res. 2012, 209794 (2012).
Qian, B. et al. PPARβ/δ activation protects against hepatic ischaemia-reperfusion injury. Liver Int.43, 2808–2823 (2023).
Rada, P. et al. WNT-3A regulates an Axin1/NRF2 complex that regulates antioxidant metabolism in hepatocytes. Antioxid. Redox Signal. 22, 555–571 (2015).
Dong, J. et al. SRY is a key mediator of sexual dimorphism in hepatic ischemia/reperfusion injury. Ann. Surg. 276, 345–356 (2022).
Wang, Y. et al. Axin1 participates in blood-brain barrier protection during experimental ischemic stroke via phosphorylation at Thr485 in rats. J. Chem. Neuroanat. 127, 102204 (2023).
Wang, H. et al. Tankyrase inhibition attenuates cardiac dilatation and dysfunction in ischemic heart failure. Int J. Mol. Sci. 23, 10059 (2022).
Yu, L. et al. Atorvastatin promotes pro/anti-inflammatory phenotypic transformation of microglia via Wnt/β-catenin pathway in hypoxic-ischemic neonatal rats. Mol. Neurobiol. 24, https://doi.org/10.1007/s12035-12023-03777-y (2023).
Choi, E. J. et al. Axin1 expression facilitates cell death induced by aurora kinase inhibition through PARP activation. J. Cell Biochem. 112, 2392–2402 (2011).
Xu, H. et al. AXIN1 protects against testicular germ cell tumors via the PI3K/AKT/mTOR signaling pathway. Oncol. Lett. 14, 981–986 (2017).
Zhang, G. et al. HIF-1α/microRNA-128-3p axis protects hippocampal neurons from apoptosis via the Axin1-mediated Wnt/β-catenin signaling pathway in Parkinson’s disease models. Aging 12, 4067–4081 (2020).
Figeac, N. & Zammit, P. S. Coordinated action of Axin1 and Axin2 suppresses β-catenin to regulate muscle stem cell function. Cell. Signal. 27, 1652–1665 (2015).
Albrecht, L. V., Tejeda-Muñoz, N. & De Robertis, E. M. Cell biology of canonical Wnt signaling. Annu. Rev. Cell Dev. Biol. 37, 369–389 (2021).
Callow, M. G. et al. Ubiquitin ligase RNF146 regulates tankyrase and Axin to promote Wnt signaling. PLoS ONE 6, e22595 (2011).
Sun, Y. & Li, Z. J. The multifunctional adaptor protein HIP-55 couples Smad7 to accelerate TGF-β type I receptor degradation. Acta Pharmacol. Sin. 43, 634–644 (2022).
Liu, W. et al. Axin is a scaffold protein in TGF-beta signaling that promotes degradation of Smad7 by Arkadia. EMBO J. 25, 1646–1658 (2006).
Gao, R. et al. Rnf25/AO7 positively regulates wnt signaling via disrupting Nkd1-Axin inhibitory complex independent of its ubiquitin ligase activity. Oncotarget 7, 23850–23859 (2016).
Dao, K. H. et al. The PI3K/Akt1 pathway enhances steady-state levels of FANCL. Mol. Biol. Cell 24, 2582–2592 (2013).
Groiss, S. et al. Inter-patient heterogeneity in the hepatic ischemia-reperfusion injury transcriptome: implications for research and diagnostics. New Biotechnol. 79, 20–29 (2024).
Acknowledgements
This work was jointly supported by grants from the Natural Science Foundation of Heilongjiang Province of China (LC2018037 to Y.M.), Outstanding Youth Training Fund from Academician Yu Weihan of Harbin Medical University (2014), Scientific Foundation of the First Affiliated Hospital of Harbin Medical University (HYD2020JQ0007 and 2019L01 to Y.M.), The National Natural Scientific Foundation of China (82370643, 81100305 and 81470876 to Y.M.), Heilongjiang Postdoctoral Foundation (LBH-Q17097 and LBH-Z11066 to Y.M.), and China Postdoctoral Science Foundation (2012M510990 and 2013T60387 to Y.M.).
Author information
Authors and Affiliations
Contributions
B.Q. and Y.M. performed study concept and design. B.Y. and H.Y. performed experiments. C.W., S.L., S.K., Z.L., X.L., Y.H., Z.L., Y.Z., Z.M., and Y.F. analyzed data, discussed results, and provided important intellectual content throughout the study. B.Q., H.Y., W.T., and Y.M. wrote the paper. The order of authorship was determined by overall contributions and approved by all the authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks the anonymous, reviewers for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Qian, B., Yin, B., Yu, H. et al. Axin formation inhibitor 1 aggravates hepatic ischemia‒reperfusion injury by promoting the ubiquitination and degradation of PPARβ. Nat Commun 16, 1776 (2025). https://doi.org/10.1038/s41467-025-56967-8
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41467-025-56967-8