Abstract
Senescence-associated frailty and sarcopenia are global challenges. We here investigated neuronal activity and skeletal muscle biology in senescence-accelerated mouse prone 8 (SAMP8) mice with scalp acupuncture stimulation (SAPS). Excise activity was assessed using rotarod test in the three groups: SAMP8 mice receiving SAPS (SP8-Ap), SAMP8 controls (SP8-C), and senescence-accelerated mouse resistant 1 controls (SR1). SP8-Ap exhibited significantly improved exercise activity compared to SP8-C. SAPS increased brain-derived neurotrophic factor (BDNF) and nerve growth factor (NGF) levels in the frontal cortex of SP8-Ap. Monocyte/macrophage infiltration was significantly reduced in the gastrocnemius of SP8-Ap mice, which was associated with reduced expression of various glycogen synthase kinase-3β (GSK3β)-mediated inflammatory genes and increased insulin-like growth factor (IGF)-1 mRNA and phosphorylated AKT. These results indicate that elevation of neurotropic factors in the frontal cortex by SAPS can improve the exercise activity and skeletal muscle status. SAPS may represent a novel therapeutic approach to improve senescence-related frailty.
Similar content being viewed by others
Introduction
In the twenty-first century, life expectancy has increased significantly within the overall population1. Frailty and sarcopenia have emerged as urgent social and economic problem worldwide. Frailty is a prevalent clinical syndrome in elderly, characterized by a decline in physiological reserves across multiple systems. It is associated with an increased risk of adverse health outcomes including falls, disability, hospitalization, and mortality2. Frailty encompasses a multifaceted spectrum, encompassing physical factors such as muscle weakness, mental and psychological factors such as dementia and depression, and social factors such as social isolation and financial hardship. The senescence-related loss of skeletal muscle mass, named primary sarcopenia, is associated with a wide range of adverse effects on healthy life such as impaired physical activity, poor quality of life and death3,4. Therefore, developing effective interventions to combat senescence-associated frailty and sarcopenia is a critical global challenge for extending healthy lifespan.
Inflammatory response plays a critical role in skeletal muscle degeneration and regeneration. Pro-inflammatory cytokines, such as tumor necrosis factor -α (TNF-α), interleukin (IL)-1βand IL-6, are elevated in the circulation of elderly individuals compared to young healthy adults5. IL-6 and TNF-α have been shown to exacerbate skeletal muscle degradation in rats6,7. Notably, TNF-α activates caspases, leading to apoptosis in skeletal muscle cells through receptor binding8. Apoptotic cells are subsequently phagocytosed by macrophages. Infiltration of macrophages into skeletal muscle is increased in patients with acute muscle atrophy aged 50-59 years9. Classically activated macrophages (M1 phenotype) release pro-inflammatory cytokines (TNF-α, IL-1β and IL-6) and chemokines, such as C-C motif chemokine 2 (CCL2), perpetuating chronic inflammation10. On the other hand, macrophages involve in tissue regeneration by the enhancing the production of insulin-like growth factor 1 (IGF1)11. IGF1 promotes muscle regeneration by binding to its receptor (IGF1R) and activating PI3K-AKT-mTOR signaling pathway12. Furthermore, phosphorylation of glycogen synthase kinase 3 (GSK3β) at Serine 9, mediated by activation of IGF1-PI3K-AKT signaling pathway, contributes to macrophage tolerance13.
Recently, sarcopenia has been linked to impairments in central nervous system function14. Previous studies have demonstrated that scalp acupuncture stimulation (SAPS) improves brain function in rats with cerebral palsy15 or intracranial hemorrhage16, and mice with cerebral artery occlusion17. Our previous research has shown an antidepressant effect of SAPS in mouse models of depression, attributed to increased expression of brain-derived neurotrophic factor (BDNF), neurotrophin (NT)-3, and NT-4/5 in the brain18,19. Neurotrophic factors are crucial for various neuronal functions, including synapse formation and elongation, regulation of synaptic function, apoptosis, and memory learning formation20,21,22. Neurotropic factors, such as BDNF and nerve growth factor (NGF), have shown to modulate neuroinflammation, which can associate with their neuroprotective effects23,24.
Given the lower risk of adverse effects compared to pharmacological therapy, acupuncture is gaining increasing attention as a potential treatment for improving health conditions, particularly in the elderly. Therefore, this study investigated the effects of SAPS on the brain and skeletal muscle using senescence-accelerated model mice.
Results
Determination of the protocol of rotarod test with acupuncture
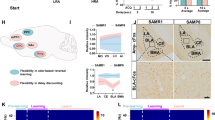
To investigate the effect of SAPS on senescence-associated frailty and sarcopenia, we used senescence-accelerated mouse prone 8 (SAMP8) and senescence-accelerated mouse resistant 1 (SAMR1) mice. SAMP8 mice exhibit accelerated aging and short lifespan, while SAMR1 mice exhibit a normal aging25. SAMP8 mice are a widely used model for studying aging process associating with learning and memory impairment26,27. The experimental protocol is outlined in Fig. 1a. Traditional acupuncture stimulation was performed at two acupuncture points on the mouse scalp, Bai-Hui (GV20), located 5 mm above the midpoint connecting the front edges of both ears, and Yintáng, located 3 mm above the midpoint connecting the from edges of eyes (Ex-HN3) (Fig. 1b). All mice underwent rotarod test, which is widely used method in rodents to evaluate exercise activity, reflecting balance, corresponding ability to impairment, and muscle tolerance to exercise28. To determine the pre-training condition, we optimized a constant speed of the rotating rod (Supplementary Fig. 1). Mice were initially placed on a rotarod rotating at 5 resolutions per minute (rpm). At this speed, most mice were unable to maintain balance and fell off. Conversely, at 15 rpm, mice struggled to maintain balance from the beginning and quickly gave up. Based on these observations, 10 rpm was determined to be an appropriate pre-training speed (Fig. 1c). All mice underwent a pre-training period on the rotarod at a constant speed of 10 rpm for 3 minutes, five days per week (Monday to Friday) during week 22. Subsequently, the rotarod test was performed three times per week (weeks 23-25). In this test, the rotarod speed gradually increased from 4 to 40 rpm over 10 min, following a commonly used protocol29,30 (Fig. 1d).
a Protocol of rotarod test and intervention. In SP8-Ap, scalp acupuncture stimulation was performed three times a week at 24 and 25 weeks. Each stimulation was followed by rotarod test. b Points of scalp acupuncture, Bai-Hui (GV20) and Yintáng (Ex-HN3). c Pictures of a mouse running on the rotating rod machine and the speed of rotating rod machine at 10 rpm in pre-training. rpm, resolutions per minute. d The speed of rotating rod machine in rotarod test. rpm, resolutions per minute. SP8-Ap, senescence-accelerated prone mice 8 received acupuncture stimulation; SP8-C, senescence-accelerated prone mice 8 without acupuncture stimulation; SR1, senescence-accelerated resistant mice 1 without acupuncture stimulation.
Body weight, skeletal muscle mass and rotarod test
Body weight of SP8-C mice was significantly lower than that of SR1 mice at 23, 24 and 25 weeks (p < 0.01). While the body weight of SP8-Ap mice tended to be higher than that of SP8-C mice at 23, 24 and 25 weeks, no statistically significant difference was observed between these groups (Fig. 2a). Skeletal muscle mass, assessed as the ratio of gastrocnemius muscle weight to total body weight, was significantly reduced in SP8 mice compared to SR1 mice at 25 weeks (SP8-C vs. SR1, p < 0.05, r = 0.66; SP8-Ap vs. SR1, p < 0.05, r = 0.42). No significant differences in muscle mass were observed between SP8-C and SP8-Ap groups (Fig. 2b).
a Body weight of SR1, SP-C and SP8-Ap mice at 22, 23, 24 and 25 weeks. b Gastrocnemius muscle mass of SR1, SP-C and SP8-Ap mice at 25 weeks. c Time to fall in rotarod test. Each time to fall in SR1, SP8-C and SP8-Ap was plotted as open circle, closed triangle and closed circle, respectively. SP8-Ap, senescence-accelerated prone mice 8 received acupuncture stimulation; SP8-C, senescence-accelerated prone mice 8 without acupuncture stimulation; SR1, senescence-accelerated resistant mice 1 without acupuncture stimulation.
Rotarod test demonstrated a significant improvement in time to fall in SP8-Ap mice throughout the SAPS treatment period (from 23 to 25 weeks) (p < 0.05, r = 0.69). Additionally, time to fall at 25 weeks was significantly longer compared to that at 23 weeks within the SP8-Ap group (p < 0.05, r = 0.58) (Fig. 2c). These findings indicate that exercise activity was significantly improved by SAPS in senescence-accelerated mice.
Increased expression of neurotrophic factors in the prefrontal cortex by scalp aquapuncture stimulation
The observed improvement in exercise activity in SR8-Ap mice prompted further investigation into the expression of neurotrophic factors and cytokines in the prefrontal cortex of brain. Gene expression of Bdnf and Ngf was significantly reduced in SP8-C mice compared to SR1 mice (Bdnf, p < 0.05, r = 0.58; Ngf, p < 0.01, r = 0.70). Notably, these expressions were significantly increased in SP8-Ap mice compared to SP8-C mice (Bdnf, p < 0.05 r = 0.62; Ngf, p < 0.05 r = 0.76) (Fig. 3a).
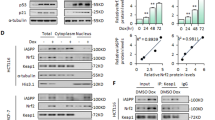
a Gene expression for Bdnf and Ngf. b Western blotting for BDNF, NGF, TrkB and TrkA. c Protein expression of BDNF, NGF, TrkA and TrkB. d Immunohistochemistry for BDNF and NGF. NeuN is a marker for nucleus of neuron. Scale bar, 50 μm. e Gene expression for Iba1, Cd68 and Gfap. f Serum BDNF levels. b-actin, beta actin; BDNF, brain-derived neurotrophic factor; Cd68, cluster of differentiation 68; Gfap, glial fibrillary acidic protein; Iba1, ionized calcium-binding adapter molecule 1, NeuN, neuronal nuclei; NGF, nerve growth factor; SP8-Ap, senescence-accelerated prone mice 8 received acupuncture stimulation; SP8-C, senescence-accelerated prone mice 8 without acupuncture stimulation; SR1, senescence-accelerated resistant mice 1 without acupuncture stimulation, Trk, tyrosine protein kinase.
Protein levels of BDNF, NGF, tyrosine protein kinase A (TrkA, the NGF receptor) and TrkB (the BDNF receptor) were examined in four representative mice from each group (Fig. 3b and (Supplementary Fig. 2) and quantified (Fig. 3c). Consistent with the gene expression data, protein levels of BDNF and NGF were decreased in SP8-C mice compared to SR1 mice, whereas these levels were significantly upregulated by SAPS in SP8-Ap mice compared to SP8-C mice (BDNF, p < 0.01, d = 3.74; NGF, p = 0.076, d = 1.21) (Fig. 3c). Furthermore, protein expression of TrkA and TrkB was also downregulated in SP8-C mice compared to SR1 mice, but their expression was significantly upregulated in SP8-Ap mice compared to SP8-C mice (TrkA, p < 0.01, d = 2.79; TrkB, p < 0.05, d = 2.79) (Fig. 3c). Each BDNF and NGF expression was co-localized with neuronal nuclei (NeuN), a neuronal marker, in all groups, indicating that these neurotrophic factors are primarily expressed within neurons (Fig. 3d). These findings suggest that SAPS promotes the elevation of neurotrophic factors, BDNF and NGF, in the prefrontal cortex, potentially contributing to neuroprotective effects.
The significantly increased Bdnf and Ngf expression in SP8-Ap led us to further exam for the cellular conditions of microglia and astrocytes. Gene expression of markers for microglia, Ionized calcium-binding adapter molecule 1 (Iba1) and cluster of differentiation 68 (Cd68), showed a trend of downregulating in SP8-Ap mice compared to SP8-C mice (Iba1, p = 0.29, d = 0.22; Cd68, p = 0.18, d = 0.31). Gene expression of glial fibrillary acidic protein (Gfap), a marker for astrocytes, tended to be reduced in SP8-C compared to SR1 (p = 0.35, r = 0.18), whereas the expression was significantly increased in SP8-Ap compared to SP8-C (p < 0.05, r = 0.62) (Fig. 3e).
Serum BDNF levels tended to be lower in SP8-C mice compared to SR1 mice, whereas this trend toward a decrease tended to be reversed weakly in SP8-Ap mice (r = 0.17) (Fig. 3f).
Scalp acupuncture stimulation potentially attenuated oxidative stress in the prefrontal cortex
We assessed the effect of SAPS on oxidative stress in the prefrontal cortex. Gene expression of Catalase (Cat) was significantly reduced (p < 0.05, d = 0.55), and the expression of Nuclear factor erythroid 2-related factor 2 (Nfe2l2) and Sirtuin 1 (Sirt1) tended to be reduced in SP8-C compared to SR1 (Sirt1, p = 0.069, d = 0.41; Nfe2l2, p = 0.144, d = 0.40), whereas the expression of these antioxidant markers tended to be upregulated in SP8-Ap mice compared to SP8-C mice (Cat, p = 0.251, d = 0.38; Sirt1, p = 0.624, d = 0.21; Nfe2l2, p = 0.125, d = 0.38) (Supplementaly Fig. 3a). Immunohistochemistry for frozen brain sections (IHC/Fr) for 4-Hydroxynonenal (4-HNE), a well- known oxidative stress marker protein, demonstrated that the expression of 4-HNE level in the neurons was increased in SP8-C compared to SR1, whereas ameliorated in SP8-Ap compared to SP8-C (Supplementaly Fig. 3b). These results suggested that SAPS potentially attenuate oxidative stress in the prefrontal cortex of senescence-accelerated mice.
Scalp acupuncture stimulation attenuates macrophage infiltration in skeletal muscle
We hypothesized that the improvement in exercise activity observed with SAPS would be associated with beneficial effects on skeletal muscle function. To investigate this, we examined the gastrocnemius muscle of each mouse.
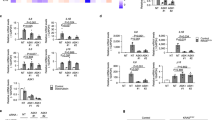
Histological analysis of the gastrocnemius muscle revealed significant reductions in both muscle fiber length and width in both SP8-C and SP8-Ap mice compared to SR1mice (length vs. SR1, SP8-C, p < 0.01 r = 0.62; SP8-Ap, p < 0.0001 r = 0.44 ; width vs. SR1, SP8-C, p < 0.0001 r = 0.60; SP8-Ap, p < 0.0001 r = 0.63), consistent with muscle atrophy in senescence-accelerated mice (Fig. 4a–b). Notably, muscle fiber length was significantly increased in SP8-Ap mice compared to SP8-C group mice (p < 0.05 r = 0.27). Furthermore, histological examination revealed increased infiltration of mononuclear cells in the gastrocnemius muscle of SP8-C mice compared to SR1 mice, whereas this infiltration was attenuated in SP8-Ap mice (Fig. 4a). Gene expression of a pro-inflammatory cell marker Integrin subunitαM (Itgam)31 and a pan-macrophage marker Epidermal growth factor-like module-containing mucin-like hormone receptor-like 1 (Emr1/Adgre1)32,33 were significantly increased in SP8-C mice compared to SR1 mice (Itgam, p < 0.05, r = 0.53; Adgre1, p < 0.05, r = 0.62). Interestingly, the expression of Adgre1 and a M1-phenotype macrophage marker Cd8034 was significantly reduced in SP8-Ap mice compared to SP8-C mice (Adgre1, p < 0.05, r = 0.55; Cd80, p < 0.05, r = 0.76) (Fig. 4c). CD11b (encoded by Itgam) is a marker for mononuclear cells that play crucial roles in various immune responses, including pathogen recognition, phagocytosis, and cell survival35. Imuunohistochemical analysis revealed increased infiltration of CD11b- and F4/80 (encoded by Adgre1)-positive cells around muscle fibers in SP8-C mice compared to SR1 mice. This increased infiltration of inflammatory cells was attenuated in SP8-Ap mice (Fig. 4d–e). These observations indicated that the infiltration of mononuclear cells including macrophages were attenuated by SAPS in skeletal muscle of senescence-accelerated mice.
a Hematoxylin and eosin staining of skeletal muscle. Scale bar, 50 μm. b Major and width of skeletal muscle cells. c Gene expression of Itgam, Adgre1 and Cd80. d Immunohistochemistry for CD11b. e Immunohistochemistry for F4/80. Scale bar, 50 μm. Adgre1, epidermal growth factor-like module-containing mucin-like hormone receptor-like 1 (EMR1, F8/40); Cd, cluster of differentiation; Itgam, integrin-α M (CD11b); SP8-Ap, senescence-accelerated prone mice 8 received acupuncture stimulation; SP8-C, senescence-accelerated prone mice 8 without acupuncture stimulation; SR1, senescence-accelerated resistant mice 1 without acupuncture stimulation.
The IGF1- AKT-GSK3β signaling pathway leads to the attenuation of muscle inflammation by scalp acupuncture stimulation in skeletal muscle
The attenuation of infiltrated inflammatory cells led us to further investigate whether energy metabolism genes were altered in SP8-Ap mice. The mRNA levels of solute carrier family 2 member 4 (Slc2a4), sterol regulatory element binding transcription factor 1 (Srebf1), fatty acid binding protein 4 (Fabp4), UDP-glucose pyrophosphorylase 2 (Ugp2), forkhead box O1 (Foxo1), protein phosphatase 1 regulatory inhibitor subunit 1 A (Ppp1r1a), mitochondrially encoded cytochrome c oxidase II (mt-Co2) and transcription factor A, mitochondrial (Tfam), and peroxisome proliferator-activated receptor α (Ppara) were significantly decreased by aging (log2[SP8C/SR1]), while there were almost no changes by SAPS (log2[SP8-ApC/SP8-C]) (Fig. 5a). Notably, the mRNA levels of peroxisome proliferator activated receptor alpha (Ppara) and insulin-like growth factor 1 (Igf1) were increased by SAPS, although these were decreased or unchanged by aging (Fig. 5a). It is known that Igf1 activates AKT signaling36. Therefore, we further examined the phosphorylation of AKT and its downstream targets, including mammalian target of rapamycin (mTOR) and glycogen synthase kinase 3-β (GSK3β) (Fig. 5b). The amount of phosphorylated AKT, indicating AKT activation, and phosphorylated GSK3β, indicating GSK3β activation, were significantly increased in SP8-Ap mice compared to SR1 mice (AKT, p < 0.01 d = 3.13; GSK3β, p < 0.05, d = 2.49) and SP8-C (AKT, p < 0.01, d = 2.47; GSK3β, p < 0.01, d = 3.45), but not mTOR activation (Fig. 5c–d and Supplementary Fig. 4). The GSK3β pathway regulates the inflammatory gene expression37. Indeed, GSK3β-mediated inflammatory genes, including interleukin 1β (Il1β), C-C motif chemokine 2 (Ccl2), interferon α (Infa), prostaglandin-endoperoxide synthase 2 (Ptgs2), Il6, matrix metalloproteinase-9 (Mmp9), C-X-C motif chemokine ligand 1 (Cxcl1), Intercellular adhesion molecule 1 (Icam1), nitric oxide synthase 2 (Nos2) and tumor necrosis factor alpha (Tnfα), were reduced in the SP8-Ap mice, suggesting that the local pro-inflammatory response was attenuated through the upregulation of p-GSK3β (Fig. 5e). These results indicate that the local pro-inflammatory response in skeletal muscle was attenuated through the Igf1-AKT-GSK3β signaling pathway by SAPS in senescence-accelerated mice.
a mRNA expression (LogFc) profile of energy metabolism genes, Slc2a4, Srebf1, Fabp4, Ugp2, Foxo1, Ppp1r1a, mt-Co2, Tfam, Ppara and Igf1. b Illustration of Igf1-AKT-GSK3β signaling pathway. c Western blotting analysis for phosphorylation of AKT, mTOR and GSK3β (Ser9). d Protein expression of AKT, mTOR and GSK3β (Ser9). e mRNA expression profiles of GSK3β-mediated inflammatory genes, Il1b, Ccl2, Infa, Ptgs2, Il6, Mmp9, Cxcl1, Icam1, Nos2 and Tnf. Ccl2, C-C motif chemokine 2; Cd, Cluster of differentiation; Cxcl1: C-X-C motif chemokine ligand 1; Fabp4, fatty acid binding protein 4; Foxo1, forkhead box O1; GSK3β: glycogen synthase kinase 3β; Icam1, intercellular adhesion molecule 1; Igf1, insulin-like growth factor 1; Il, interleukin; Infα, interferon α; Mmp9, matrix metalloproteinase-9; mt-Co2, mitochondrially encoded cytochrome c oxidase II; mTOR, mammalian target of rapamycin; Nos2, nitric oxide synthase 2; p-AKT, phosphorylated AKT, p-GSK3β (Ser9), phosphorylated GSK3β at Serine 9; p-mTOR, phosphorylated mTOR; Ppp1r1a, protein phosphatase 1, regulatory inhibitor subunit 1 A; Ptgs2, prostaglandin-endoperoxide synthase 2; Slc2a4, solute carrier family 2 member 4; SP8-Ap, senescence-accelerated prone mice 8 received acupuncture stimulation; SP8-C, senescence-accelerated prone mice 8 without acupuncture stimulation; SR1, senescence-accelerated resistant mice 1 without acupuncture stimulation, Srebf1, sterol regulatory element binding transcription factor 1; Tfam, transcription factor A, mitochondrial; Tnfa, tumor necrosis factor α; Ugp2, UDP-glucose pyrophosphorylase 2;.
To assess the effect of SAPS on myogenesis, we measured the gene expression levels of Paired box 7 (Pax7) and myosin heavy chain (MyHC) isoforms consisting of Myh7, Myh2, Myh1and Myh4. Pax7 is known to regulate the differentiation of muscle satellite cells into myoblasts and other processes related to myogenesis38. Myh7, Myh2, Myh1and Myh4 were abundant in type I, IIa, IIx and IIb muscle fiber, respectively39. The expression of Pax7 tended to be reduced (p = 0.59, r = 0.38), and that of Myh7, Myh2, Myh1and Myh4 was significantly decreased in SP8-C compared to SR1(Myh7, p < 0.001 r = 0.80; Myh2, p < 0.01 r = 0.67; Myh1 p < 0.05 r = 0.73; Myh4 p < 0.01 r = 0.76), whereas their reduced expression was not recovered in SAP8-Ap (Supplementary Fig. 5). These results indicated that SAPS did not influence the differentiation of muscle satellite cells or fiber type-specific myogenesis in this senescence-accelerated model.
In summary, elevation of neurotropic factors in the frontal cortex by SAPS may improve exercise activity and skeletal muscle status in senescence-accelerating mice (Fig. 6). SAPS could have a potential to be novel therapeutic approach for improving senescence-related frailty.
This diagram illustrates the effects of scalp acupuncture stimulation on brain and muscle function. It highlights the elevation of brain-derived neurotrophic factor (BDNF) and nerve growth factor (NGF) in the brain, leading to improved exercise activity as measured by the rotarod test. Additionally, it shows improvements in muscle condition through activation of the insulin-like growth factor (Igf1)-AKT-glycogen synthase kinase 3β (GSK3β) signaling pathway and reduction of macrophage infiltration and local inflammation.
Discussion
In this study, we demonstrated a significant improvement in exercise activity by SAPS in senescence-accelerated model mice. SAPS was significantly associated with upregulation of the neurotropic factors BDNF and NGF in the brain and attenuation of local inflammation in the gastrocnemius muscle through activation of the GSK3β signaling pathway. Sufficient physical training and nutrient intake are often difficult, particularly in elderly people with frailty and sarcopenia. SAPS can be performed on elderly people to improve exercise activity regardless of such undesirable conditions.
Previous studies have focused on direct muscle stimulation using acupuncture or a combination of acupuncture and low-frequency electric stimulation to improve exercise activity40. In a tibial nerve denervation-induced muscle atrophy model, direct muscle stimulation using acupuncture plus low-frequency electric stimulation enhanced the expression of macrophage markers (F4/80, IL-1β) and inflammatory cytokines (IL-6, IFNγ, and TNFα) to attenuate muscle atrophy41. Another study reported that motor function was restored in healthy elderly people by electrical stimulation to the area near the motor nerves of the muscles42. SAPS has been investigated only for improving brain function in various animal models15,16,17,18,19. In this study, we examined the effect of SAPS alone on the brain-muscle axis.
We demonstrated the expression of neurotrophic factors BDNF and NGF in the brain of SAMP8 was increased by SAPS. It has been shown that DL0410, an anti-Alzheimer disease (AD) agent, increased protein expression of BDNF in the brain of SAMP843. Additionally, daily intake of myricetin, a polyphenol, increased gene expression of BDNF in SAMP8, associating with improvement of cognitive function44. We showed SAPS as an additional approach to upregulate BDNF in the brain of SAMP8. In the present study, we assessed the expression of BDNF in the prefrontal cortex of the brain. The prefrontal cortex is involved in higher brain functions, including working memory, cognitive/executive function, and emotional/motivational function. BDNF is an important target to improve cognitive dysfunction, which is commonly observed in AD45. Previous studies have shown that BDNF in the prefrontal cortex was dysregulated in AD46. Additionally, a significant association of BDNF in the prefrontal cortex with working memory has been reported in Down syndrome47. We demonstrated BDNF protein was localized around the nuclei of neurons, suggesting that the impaired higher brain function of the frontal cortex can be improved by SAPS, which can result in improvement of exercise activity.
Along with BDNF, NGF is also known to be involved in cell growth, maintenance, proliferation and survival of cholinergic neurons in the central nervous system and peripheral sympathetic neurons. NGF is produced in basal forebrain cholinergic neurons to be retrogradely transported along the axons to the bodies of cholinergic neurons48. NGF binds to the high-affinity specific receptor TrkA or the low affinity receptor p75 to maintain the function of cholinergic neuron49. We revealed that upregulated NGF protein was localized around the nuclei of neurons. These results suggested that the upregulated NGF protein can exert the neuroprotective effects for neurons, which can improve the function of cholinergic neurons, leading to improvement of exercise activity in senescence-accelerated mice.
It has been shown that BDNF partially associates with downregulating proinflammatory cytokines in microglia50,51. Together, NGF has shown to counteract the amyloid beta (Aβ)-induced proinflammatory activation of microglia24. We demonstrated SAPS potentially ameliorated cellular activation of microglia and may retrieve astrocyte functions, such as blood brain barrier and supplying nutrients to neurons52, in the prefrontal cortex of senescence-accelerated mice. Additionally, we demonstrated SAPS potentially attenuated oxidative stress in the prefrontal cortex of these mice. Excessive accumulation of reactive oxygen species and activation of microglia have shown to associate with progressive cognitive impairment and neuronal degeneration in AD53,54. Given these results, it was suggested that the SAPS-induced BDNF and NGF potentially attenuated oxidative stress, which may modulate cellular condition of microglia and astrocytes, leading to ameliorate neuronal degeneration and functional damage in the prefrontal cortex of senescence-accelerated mice.
Following the examination of the brain, we investigated the effects of SAPS on skeletal muscle. In the histological analysis, it was surprising that SAPS attenuated inflammatory cell infiltration in senescence-accelerated mice. IHC/Fr analysis indicated that the major component of inflammatory cells were macrophages. Gene expression analysis for Cd80 and IHF/Fr suggested that infiltration of M1-phonotype macrophage was reduced by SAPS. The reduced macrophage number and phenotype shift from M1 to M2 can play an important role in attenuating skeletal muscle inflammation by SAPS.
Notably, we demonstrated that SAPS enhanced phosphorylation of GSK3β (Ser9), which has shown to be involved in macrophage tolerance and attenuate the inflammatory response by suppressing the production of pro-inflammatory cytokines such as IL-1β, IL-613,55. In the present study, the expression of various GSK3β-mediated inflammatory genes was reduced by SAPS, which supported the activation of IGF1-PI3K-GSK3β signaling pathway. These results suggested that SAPS can attenuate the inflammatory response in skeletal muscle by reducing the infiltration of mononuclear cells, including M1-phenotype macrophages, through the activation of the IGF1-PI3K-GSK3β signaling pathway.
It was hypothesized that the attenuated local inflammation of skeletal muscle can restore the impaired energy production even in aged skeletal muscle. We demonstrated that the expression of various genes involved in energy metabolism, which was reduced in senescence-accelerated mice, was restored by SAPS. Some of these ameliorated genes involved in mitochondrial energy production. Mitochondria modulates a vast range of biological processes, including energy production and inflammation56. SAPS can have the potential to restore mitochondrial function, including energy production, in skeletal muscle.
It was also hypothesized that the attenuated local inflammation can restore skeletal muscle cell regeneration. However, we were not able to observe an increase in skeletal muscle mass by SAPS. In addition, the mTOR signaling pathway, which is essential for muscle regeneration, was not activated in this study. Furthermore, we did not observe an increase in muscle satellite cell differentiation or fiber type-specific myogenesis by SAPS. In this senescence-accelerated model, the decline in function of muscle satellite cells and/or the impaired regenerative ability of myofibers can influence such insufficient muscle regeneration. It is well known that exercise training mediates muscle growth and adaptation57. Pre-training at 22 weeks and rotarod test at 23 to 25 weeks may help improve muscle inflammation and energy metabolism, while this physical exercise did not result in an increase in muscle regeneration in this senescence-accelerated model.
To clarify the mechanism of how brain stimulation affects distant muscles, we measured blood BDNF concentrations. Although the increase of BDNF in SP8-Ap mice was a trend, not significant, this finding suggested that BDNF increased in the brain may flow out into the blood, contributing to the improvement of skeletal muscle function.
The present study has some limitations: while we postulated that SAPS-mediated changes in neurotrophic factors contribute to muscle function, no direct causal link between CNS changes and peripheral improvements was demonstrated. The sample sizes (n = 6–9 per group) are relatively small. Larger cohorts would improve statistical robustness, particularly for ELISA-based serum BDNF measurements, which did not reach significance.
SAPS combined with electrical stimulation has shown better effects on the brain function compared to the routine SAPS in patients with motor aphagia after cerebral infarction58 or in patient with dysphagia after acute stroke59. In future studies, it will be interesting to investigate whether electrical SAPS improves exercise activity and muscle status more significantly in senescence-accelerating mice. Blood BDNF should be also assessed in this electrical SAPS model. Together, future work should explore whether extend SAPS treatment beyond 2 weeks or additional interventions (e.g., resistant training) could synergistically enhance muscle regeneration. Furthermore, muscle response to systemic administration of BDNF/NGF or TrkB/TrkA blockers would confirm the causal link between SAPS-induced neurotrophic factor elevation and muscle function improvements. In these studies, measurement of grip strength and open-field tests in rotarod test will be added to provide a more comprehensive evaluation regarding muscle performance. Neuroimaging (e.g., functional MRI and 18F-fluorodeoxyglucose positron emission tomography/computed tomography60,61) cortical electrophysiological assessments, and in vivo electromyography will provide further validation of SAPS effects on cortical excitability and its downstream neuromuscular consequences.
While the use of SAMP8 mice is appropriate for modeling accelerated aging, additional studies in naturally aged rodents and ultimately in clinical populations are necessary to assess translatability. The feasibility of SAPS in elderly patients, along with potential placebo effects, should be explored in future randomized controlled trials.
Methods
Experimental animals
To eliminate the influence of the estrous cycle, male senescence-accelerated mouse prone 8 (SAMP8) and male senescence-accelerated mouse resistant 1 (SAMR1) mice were obtained from CLEA Japan, Inc. (Tokyo, Japan). All animals were housed in a controlled environment with a 12 h light/dark cycle at 22 ± 3 °C and 55 ± 5% humidity with free access to food and water throughout the experiment. We conducted the experiments in three groups of mice: SAMP8 received acupuncture stimulation (SP8-Ap, n = 8), SAMP8 control without acupuncture stimulation (SP8-C, n = 6) and SAMR1 without acupuncture stimulation (SR1, n = 9). All experimental procedures were performed according to the National Institute of Health Guide for the Care and Use of Laboratory Animals and all protocols were approved by the Suzuka University Medical Science Board Committee for Animal Care and Use of Laboratory Animals (permission number: 298).
Scalp acupuncture stimulation (SAPS)
Traditional acupuncture stimulation was performed to the acupuncture points of mouse scalp, Bai-Hui (GV20) and Yintáng (Ex-HN3), as previously described18. The location of GV20 and Ex-HN3 were shown in Fig. 1b. In brief, SAPS were performed on SP8-Ap 5 times a week for 2 weeks (Monday to Friday at 24 and 25 weeks), in which stainless-steel needles (Acupuncture Needle D-Type, diameter: 0.25 mm; length: 15 mm; SEIRIN Co., Ltd., Shizuoka, Japan) were inserted to a depth of 5 mm at both GV20 and Ex-HN3 for 20 min. During acupuncture stimulation, the mouse was restrained with adhesive tape on its tail in an overturned cage. SR1 and SP8-C mice were also restrained for the same time without acupuncture stimulation.
Rotarod test
All mice underwent rotarod test, which is widely used in rodents to assess exercise activity, reflecting balance, corresponding ability to impairment, and muscle tolerance to exercise28.
A rotarod machine (ROTA-ROD/RS, LE8300, LSI LETICA Scientific instruments; Panlab, Barcelona, Spain) was used in this study (Fig. 1c). All mice were pre-trained to run on the rotating rod with a constant speed of 10 resolutions per minute (rpm) for 3 min from Monday to Friday of 22 weeks to adapt rotarod machine. Rotarod test was performed 3 times per a week on all mice during 23 to 25 weeks. In this test, the mice were run on the rotating rod with gradually increased speed from 4 to 40 rpm within 10 minutes (Fig. 1d). The time when the mice fell off the rod rotating was recorded. At 23, 24 and 25 weeks, rotarod test was completed within 30 minutes after acupuncture stimulation in SP8-Ap group. On the other hand, rotarod test was completed within 30 min after releasing from body fixation in SR1 and SP8-C groups. Data are presented as the average time to fall in each week.
Sample collection
All mice were euthanized by intraperitoneal injection of pentobarbital sodium solution (Somnopentyl, Kyoritsu Seiyaku Co., Ltd., Tokyo, Japan, 120 mg/kg) after the last rotarod test at 25 weeks. Blood was collected and centrifuged at 12,000 rpm for 5 min, thereafter supernatant was collected and saved at −80 °C until analysis.
The brains and gastrocnemius muscles were immediately collected after the euthanasia. The right prefrontal cortex and right gastrocnemius muscle were used for quantitative polymerase chain reaction (qPCR) and Western blotting. The left prefrontal cortex and left muscle were subjected to histological analyses.
Gene expression in the brain and gastrocnemius muscles
Total RNA was isolated from the brain or gastrocnemius muscle using TrizolTM Reagent (Thermo Fisher Scientific Inc., Waltham, MA) according to the manufacturer’s instructions. The cDNA was synthesized from total RNA using a cDNA Synthesis kit (Takara, Shiga, Japan). Real-time PCR quantification was performed using the PowerUpTM SYBR Green Master Mix (Thermo Fisher) and the 7300 Real-time PCR Detection System (Thermo Fisher). The PCR primer sets used for amplifying target gene were listed in Supplementary Table 1. Mean values of mRNA were normalized to Beta 2 microglobulin (B2m).
Western blotting analysis of tissues from brain and gastrocnemius muscles
Brain and skeletal muscle tissues were homogenized in RIPA lysis buffer (150 mM NaCl, 1.0% NP-40, 1% sodium deoxycholate, 0.1% sodium dodecyl sulfate, 50 mM Tris-HCl pH8.0) containing a protease inhibitor cocktail (Sigma-Aldrich, Tokyo, Japan). 15 μg of the homogenized tissues were resolved in TGXTM precast gels (Bio Rad, Hercules, CA) and transferred to polyvinylidene difluoride membrane (Bio Rad). Blotted membranes were incubated with Can Get signal solution (TOYOBO, OSAKA, Japan) as blocking reagent and primary antibodies, followed by peroxidase-conjugated secondary antibody (GE Healthcare Life Sciences, Pittsburgh, PA) in Can Get solutions. Anti-AKT (#4691, Cell Signaling Technology, Danvers, MA, USA, 1:1000), anti-phosphorylated AKT (p-AKT, #4060, Cell Signaling Technology, 1:1000), anti- BDNF (EPR1292, Abcam, Cambridge, UK, 1: 1000), anti-GSK3β (#12456, Cell Signaling Technology, 1:1000), anti-phosphorylated GSK3β at Serine 9 (p-GSK3β (Ser9), #9323 Cell Signaling Technology, 1:1000), anti-mTOR (#2983, Cell Signaling Technology, 1:1000) anti-phosphorylated mTOR (#5536, Cell Signaling Technology, 1:1000), anti-NGF (MA5-32067; Thermo Fisher Scientific, Tokyo, Japan, 1:1000), anti-TrkA (ANT-018; Thermo Fisher, 1:200) and anti-Trk B (ab187041, Abcam, 1:1000) were used as primary antibodies. The membrane was treated with Stripping buffer (Nakalai tesque, Kyoto, Japan) or Tris buffered saline with Tween 20 (TBST) with 0.02% sodium azide to remove HRP. Protein bands were visualized using enhanced chemiluminescence reagents (BioRad) and digitized using a Lumino-image analyzer (LAS4000 mini, Fuji Film, Tokyo, Japan). Expression intensity was quantified by Multi Gauge software (Fuji).
Histological analysis
IHC/Fr for BDNF, NGF and 4-HNE was performed as following. The left lobe of brain was washed with cold (4 °C) phosphate buffered saline (PBS) and were soaked in 10% sucrose solution for overnight at 4 °C at day 1. They were then washed with cold PBS again and were soaked in 20% or 30% sucrose solution for overnight at 4°C at day 2 or 3, respectively. At day 4, they were washed with cold PBS and embedded in optimal cutting temperature compound (OTC, Sakura Finetek Co., Ltd., Tokyo, Japan) to be frozen and stored at −80 °C. The frozen tissues were sectioned into 10 μm thick slices using a cryostat (Leica Biosystems.com, Tokyo, Japan). Slides were brought to room temperature before being rehydrated with PBS. Sections were then permeabilized and blocked with PBS containing 10% serum goat (Vector Laboratories, Newark, CA, USA) and 0.3% Triton-X-100 (Nakalai tesque). Brain sections were incubated with the primary antibody (Anti-BDNF (ZRB1136; Sigma-Aldrich, 1:100), anti-NGF (MA5-32067; Thermo Fisher, 1:50) or anti 4-HNE (STA-035; Cell Biolabs, Inc., San Diego, CA, USA, 1:100) plus anti-NeuN (MAB377; Merck, Rahway, NJ, 1:200) at 4 °C for overnight, washed with PBS and then incubated with secondary antibodies (Alexa 488 anti-mouse IgG and Alexa 594 anti-rabbit IgG, Thermo Fisher Scientific) for 2 h at room temperature in the dark. After washing with PBS, brain sections were incubated with Vector TrueVIEW Autofluoresence Quenching kit with DAPI (Vector Laboratories) according to the manufacturer’s instructions.
The frozen sections of gastrocnemius muscle sections were subjected to hematoxylin and eosin (HE) staining and IHC/Fr. IHC/Fr for F4/80 and CD11b were performed according to the similar protocol described above. Primary antibodies used for the muscle sections were anti-F4/80 (MCA497, Bio-Rad, 1:500) and anti-CD11b (M1/70、Biolegend, 1:100). Secondary antibodies were Alexa 488 and Alexa 564 anti-rat IgG (Thermo Fisher Scientific), respectively. Images of fluorescence were taken using a confocal scanning laser microscope (DMi8, Leica Microsystems, Tokyo, Japan).
Enzyme-Linked Immunosorbent Assay (ELISA) assay for BDNF protein in the circulating blood
Serum levels of BDNF protein were measured in duplicate using an ELISA kit (Mature BDNF ELISA Kit Wako, FUJIFILM Wako Pure Chemical Corporation, Osaka, Japan) according to the manufacturer’s instructions. Briefly, a 96-well microplate coated with anti-BDNF antibody was incubated with 4-fold diluted plasma sample at 2 h at room temperature with horizontal shaking. After the incubation, the wells were washed with a washing buffer 4 times and incubated with biotin conjugated antibody solution for 1 h at room temperature. After washing 4 times, they were incubated in peroxidase-conjugated streptavidin solution for 30 min at room temperature. After washing 4 times again, a luminescent reagent mixture was added, and the luminescence intensity was measured using a microplate reader (Thermo Fisher).
Statistical analysis
Statistical analysis of the data and visualization were performed using Prism 9 (GraphPad Software, Inc, CA, USA) or Multivariable Analysis for Mac version 3.0 software (ESUMI Co., Ltd., Tokyo, Japan). Values of gene and protein expression were expressed as means with standard error. In rotarod test, values of time to fall during 23 to 25 weeks within the same group were compared using the Kruskal-Wallis test. Values of gene or protein expression between two groups were compared using the Mann–Whitney U test or one-way ANOVA test, respectively. All tests were two-tailed, and p values of less than 0.05 were considered statistically significant. Along with p values, effective sizes were calculated. Effective sizes in parametric or nonparametric analysis were expressed as Cohen’s d or r, respectively. Values of d equal or more than 0.2 and less than 0.5, equal or more than 0.5 and less than 0.8, and equal or more than 0.8 were evaluated as small, medium and large effect, respectively. Values of r equal or more than 0.1 and less than 0.3, equal or more than 0.3 and less than 0.5, equal or more than 0.5 were evaluated as small, medium and large effect, respectively.
Data availability
All data supporting the findings of this study are available within the paper and its Supplementary Information. All primer sequences are provided in Supplementary Table 1.
References
Christensen, K., Doblhammer, G., Rau, R. & Vaupel, J. W. Ageing populations: the challenges ahead. Lancet 374, 1196–208 (2009).
Xue, Q. L. The frailty syndrome: definition and natural history. Clin. Geriatr. Med. 27, 1–15 (2011).
Cruz-Jentoft, A. J. et al. Sarcopenia: European consensus on definition and diagnosis: report of the European Working Group on Sarcopenia in Older People. Age Ageing 39, 412–23 (2010).
Sayer, A. A. & Cruz-Jentoft, A. Sarcopenia definition, diagnosis and treatment: consensus is growing. Age Ageing 51, afac220 (2022).
Nishikawa, H., Fukunishi, S., Asai, A., Yokohama, K., Nishiguchi, S. & Higuchi, K. Pathophysiology and mechanisms of primary sarcopenia (Review). Int. J. Mol. Med. 48, 48 (2021).
Goodman, M. N. Tumor necrosis factor induces skeletal muscle protein breakdown in rats. Am. J. Physiol. 260, E727–30 (1991).
Goodman, M. N. Interleukin-6 induces skeletal muscle protein breakdown in rats. Proc. Soc. Exp. Biol. Med. 205, 182–5 (1994).
Dupont-Versteegden, E. E. Apoptosis in muscle atrophy: relevance to sarcopenia. Exp. Gerontol. 40, 473–81 (2005).
Puthucheary, Z. A. et al. Acute skeletal muscle wasting in critical illness. JAMA 310, 1591–600 (2013).
Tornatore, L., Thotakura, A. K., Bennett, J., Moretti, M. & Franzoso, G. The nuclear factor kappa B signaling pathway: integrating metabolism with inflammation. Trends Cell Biol. 22, 557–66 (2012).
Vannella, K. M. & Wynn, T. A. Mechanisms of organ injury and repair by macrophages. Annu. Rev. Physiol. 79, 593–617 (2017).
Schiaffino, S., Reggiani, C., Akimoto, T. & Blaauw, B. Molecular mechanisms of skeletal muscle hypertrophy. J. Neuromuscul. Dis. 8, 169–83 (2021).
Park, S. H., Park-Min, K. H., Chen, J., Hu, X. & Ivashkiv, L. B. Tumor necrosis factor induces GSK3 kinase-mediated cross-tolerance to endotoxin in macrophages. Nat. Immunol. 12, 607–15 (2011).
Ito, N. et al. Slc12a8 in the lateral hypothalamus maintains energy metabolism and skeletal muscle functions during aging. Cell Rep. 40, 111131 (2022).
Wang, Z., Fan, X., Chen, K., Yu, X. & Gao, J. Effects of three kinds of head acupuncture therapies on regulation of brain microenvironment and rehabilitation of nerve function in rats with cerebral palsy. J. Tradit. Chin. Med. 41, 276–83 (2021).
Li, M. Y. et al. Scalp acupuncture protects against neuronal ferroptosis by activating the p62-Keap1-Nrf2 pathway in rat models of intracranial haemorrhage. J. Mol. Neurosci. 72, 82–96 (2022).
Park, H. K., Song, M. K., Kim, W. I. & Han, J. Y. Regulation of gene expression after combined scalp acupuncture and transcranial magnetic stimulation in middle cerebral artery occlusion mice. Restor. Neurol. Neurosci. 38, 253–63 (2020).
Kawanokuchi, J. et al. Acupuncture treatment for social defeat stress. Front Behav. Neurosci. 15, 685433 (2021).
Yamamoto, T. et al. Antidepressant effects of acupuncture in a murine model: regulation of neurotrophic factors. Acupunct. Med. 41, 38–47 (2023).
Allen, S. J., Watson, J. J., Shoemark, D. K., Barua, N. U. & Patel, N. K. GDNF, NGF and BDNF as therapeutic options for neurodegeneration. Pharmacol. Ther. 138, 155–75 (2013).
Colucci-D’Amato, L., Speranza, L., Volpicelli, F. Neurotrophic factor BDNF, physiological functions and therapeutic potential in depression, neurodegeneration and brain cancer. Int. J. Mol. Sci. 21, 7777 (2020).
Ibrahim, A. M., et al. Brain-derived neurotropic factor in neurodegenerative disorders. Biomedicines 10, 1143 (2022).
Dadkhah, M., Baziar, M. & Rezaei, N. The regulatory role of BDNF in neuroimmune axis function and neuroinflammation induced by chronic stress: a new therapeutic strategies for neurodegenerative disorders. Cytokine 174, 156477 (2024).
Rizzi, C. et al. NGF steers microglia toward a neuroprotective phenotype. Glia 66, 1395–416 (2018).
Reale, M., et al. Proteomic signature and mRNA expression in hippocampus of SAMP8 and SAMR1 mice during aging. Int. J. Mol. Sci. 23, 15097 (2022).
Liu, B., Liu, J. & Shi, J. S. SAMP8 mice as a model of age-related cognition decline with underlying mechanisms in Alzheimer’s disease. J. Alzheimers Dis. 75, 385–95 (2020).
Grinan-Ferre, C., Corpas, R., Puigoriol-Illamola, D., Palomera-Avalos, V., Sanfeliu, C. & Pallas, M. Understanding epigenetics in the neurodegeneration of Alzheimer’s disease: SAMP8 mouse model. J. Alzheimers Dis. 62, 943–63 (2018).
Shan, H. M., Maurer, M. A. & Schwab, M. E. Four-parameter analysis in modified Rotarod test for detecting minor motor deficits in mice. BMC Biol. 21, 177 (2023).
Liao, S. L., Lin, Y. W. & Hsieh, C. L. Neuronal regeneration after electroacupuncture treatment in ischemia-reperfusion-injured cerebral infarction rats. Biomed. Res. Int. 2017, 3178014 (2017).
Chung, J., Park, H. S., Kim, Y. J., Yu, M. H., Park, S. & Jung, S. I. Association of hepatic steatosis index with nonalcoholic fatty liver disease diagnosed by non-enhanced CT in a screening population. Diagnostics (Basel) 11, 2168 (2021).
Halfon, M., et al. ITGAM rs1143679 variant in systemic lupus erythematosus is associated with increased serum calcification propensity. Genes (Basel) 14, 1105 (2023).
Waddell, L. A. et al. ADGRE1 (EMR1, F4/80) is a rapidly-evolving gene expressed in mammalian monocyte-macrophages. Front Immunol. 9, 2246 (2018).
Legrand, F. et al. The eosinophil surface receptor epidermal growth factor-like module containing mucin-like hormone receptor 1 (EMR1): a novel therapeutic target for eosinophilic disorders. J. Allergy Clin. Immunol. 133, 1439–47, 47 e1-8 (2014).
Cutolo, M., Campitiello, R., Gotelli, E. & Soldano, S. The role of M1/M2 macrophage polarization in rheumatoid arthritis synovitis. Front Immunol. 13, 867260 (2022).
Khan, S. Q., Khan, I. & Gupta, V. CD11b activity modulates pathogenesis of lupus nephritis. Front. Med. ((Lausanne)) 5, 52 (2018).
Stitt, T. N. et al. The IGF-1/PI3K/Akt pathway prevents expression of muscle atrophy-induced ubiquitin ligases by inhibiting FOXO transcription factors. Mol. Cell 14, 395–403 (2004).
Cortes-Vieyra, R., Silva-Garcia, O., Gomez-Garcia, A., Gutierrez-Castellanos, S., Alvarez-Aguilar, C. & Baizabal-Aguirre, V. M. Glycogen synthase kinase 3beta modulates the inflammatory response activated by bacteria, viruses, and parasites. Front. Immunol. 12, 675751 (2021).
Sincennes, M. C. et al. Acetylation of PAX7 controls muscle stem cell self-renewal and differentiation potential in mice. Nat. Commun. 12, 3253 (2021).
Schiaffino, S. & Reggiani, C. Fiber types in mammalian skeletal muscles. Physiol. Rev. 91, 1447–531 (2011).
Su, Z. et al. Acupuncture plus low-frequency electrical stimulation (Acu-LFES) attenuates denervation-induced muscle atrophy. J. Appl. Physiol. (1985) 120, 426–36 (2016).
Andersen, C. J., Murphy, K. E. & Fernandez, M. L. Impact of obesity and metabolic syndrome on immunity. Adv Nutr 7, 66–75 (2016).
Di Filippo, E. S. et al. Neuromuscular electrical stimulation improves skeletal muscle regeneration through satellite cell fusion with myofibers in healthy elderly subjects. J. Appl. Physiol. (1985) 123, 501–12 (2017).
Lian, W. W. et al. DL0410 ameliorates cognitive disorder in SAMP8 mice by promoting mitochondrial dynamics and the NMDAR-CREB-BDNF pathway. Acta Pharmacol. Sin 42, 1055–68 (2021).
Shimada, Y., Sato, Y., Kumazoe, M., Kitamura, R., Fujimura, Y. & Tachibana, H. Myricetin improves cognitive function in SAMP8 mice and upregulates brain-derived neurotrophic factor and nerve growth factor. Biochem. Biophys. Res. Commun. 616, 33–40 (2022).
Amidfar, M., de Oliveira, J., Kucharska, E., Budni, J. & Kim, Y. K. The role of CREB and BDNF in neurobiology and treatment of Alzheimer’s disease. Life Sci. 257, 118020 (2020).
Aarons, T., Bradburn, S., Robinson, A., Payton, A., Pendleton, N. & Murgatroyd, C. Dysregulation of BDNF in prefrontal cortex in Alzheimer’s disease. J Alzheimers Dis 69, 1089–97 (2019).
Bimonte-Nelson, H. A., Hunter, C. L., Nelson, M. E. & Granholm, A. C. Frontal cortex BDNF levels correlate with working memory in an animal model of Down syndrome. Behav. Brain Res. 139, 47–57 (2003).
Isaev, N. K., Stelmashook, E. V. & Genrikhs, E. E. Role of nerve growth factor in plasticity of forebrain cholinergic neurons. Biochemistry 82, 291–300 (2017).
Williams, B. J., Eriksdotter-Jonhagen, M. & Granholm, A. C. Nerve growth factor in treatment and pathogenesis of Alzheimer’s disease. Prog. Neurobiol. 80, 114–28 (2006).
Lai, S. W. et al. Regulatory effects of neuroinflammatory responses through brain-derived neurotrophic factor signaling in microglial cells. Mol. Neurobiol. 55, 7487–99 (2018).
Di Cesare, M. et al. The epidemiological burden of obesity in childhood: a worldwide epidemic requiring urgent action. BMC Med. 17, 212 (2019).
Siracusa, R., Fusco, R. & Cuzzocrea, S. Astrocytes: role and functions in brain pathologies. Front. Pharmacol. 10, 1114 (2019).
Ionescu-Tucker, A. & Cotman, C. W. Emerging roles of oxidative stress in brain aging and Alzheimer’s disease. Neurobiol. Aging 107, 86–95 (2021).
Liddelow, S. A. et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature 541, 481–7 (2017).
Doble, B. W. & Woodgett, J. R. GSK-3: tricks of the trade for a multi-tasking kinase. J. Cell Sci. 116, 1175–86 (2003).
Riley, J. S. & Tait, S. W. Mitochondrial DNA in inflammation and immunity. EMBO Rep. 21, e49799 (2020).
Qaisar, R., Bhaskaran, S. & Van Remmen, H. Muscle fiber type diversification during exercise and regeneration. Free Radic. Biol. Med. 98, 56–67 (2016).
Lou, X. Q., Liu, X., Liu, C. H., Lin, H. J., Liu, H. & Ling, J. Therapeutic effect of electric-balance stimulation with scalp acupuncture for motor aphasia after cerebral infarction. Zhongguo Zhen Jiu 41, 1211–5 (2021).
Chang, L., He, P. L., Zhou, Z. Z. & Li, Y. H. Efficacy observation of dysphagia after acute stroke treated with acupuncture and functional electric stimulation]. Zhongguo Zhen Jiu 34, 737–40 (2014).
Tsurugizawa, T. et al. Awake functional MRI detects neural circuit dysfunction in a mouse model of autism. Sci. Adv. 6, eaav4520 (2020).
Shah, D., Deleye, S., Verhoye, M., Staelens, S. & Van der Linden, A. Resting-state functional MRI and [18F]-FDG PET demonstrate differences in neuronal activity between commonly used mouse strains. Neuroimage 125, 571–7 (2016).
Acknowledgements
This work was supported by Japan Society for the Promotion Science (JSPS) KAKENHI Grant Number 24K14762 to N.N.
Author information
Authors and Affiliations
Contributions
N.N., A.E., J.O. and Y.K. contributed to study concept and design, interpretation of data and drafting of the manuscript. N.N., A.E., M.N., J.K., T.Y., M.T., K.I. and Y.K. contributed to the acquisition of data. M.I. and H.N. contributed to study supervision. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Nagaoka, N., Eguchi, A., Ma, N. et al. Stimulation at the frontal cortex influences the exercise activity and skeletal muscle status in senescence-accelerating mice. npj Aging 11, 94 (2025). https://doi.org/10.1038/s41514-025-00285-2
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41514-025-00285-2