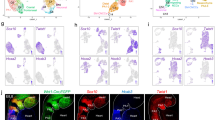
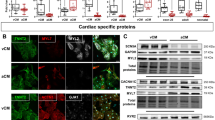
Abstract
Understanding how the atrial and ventricular heart chambers maintain distinct identities is a prerequisite for treating chamber-specific diseases. In this study, we selectively knocked out the transcription factor Tbx5 in the atrial working myocardium to evaluate its requirement for atrial identity. Atrial Tbx5 inactivation downregulated atrial cardiomyocyte (aCM)-selective gene expression. Using concurrent single-nucleus transcriptome and open chromatin profiling, genomic accessibility differences were identified between control and Tbx5 knockout aCMs, revealing that 69% of the control-enriched ATAC regions were bound by TBX5. Genes associated with these regions were downregulated in knockout aCMs, suggesting that they function as TBX5-dependent enhancers. Comparing enhancer chromatin looping using H3K27ac HiChIP identified 510 chromatin loops sensitive to TBX5 dosage, and 74.8% of control-enriched loops contained anchors in control-enriched ATAC regions. Together, these data demonstrate that TBX5 maintains the atrial gene expression program by binding to and preserving the tissue-specific chromatin architecture of atrial enhancers.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
High-throughput data used in this manuscript are available from the Gene Expression Omnibus, accession number GSE222970. Other datasets used were obtained from GSE215065 (ref. 3), GSE129503 (ref. 17) and GSE195905 (ref. 52). All other data supporting the findings in this study are included in the main article and associated files. Source data are provided with this manuscript.
Code availability
Analysis was performed using standard R packages. No custom analysis software was created to perform these analyses. Analyses were performed using published R packages that are cited within the text and the Methods section.
Change history
31 October 2023
A Correction to this paper has been published: https://doi.org/10.1038/s44161-023-00373-0
References
Mulder, B. J. M. & van der Wall, E. E. Size and function of the atria. Int. J. Cardiovasc. Imaging 24, 713–716 (2008).
Chung Mina, K. et al. Atrial fibrillation. J. Am. Coll. Cardiol. 75, 1689–1713 (2020).
Cao, Y. et al. In vivo dissection of chamber selective enhancers reveals estrogen-related receptor as a regulator of ventricular cardiomyocyte identity. Circulation. 147, 881–896 (2023).
Funakoshi, S. et al. Generation of mature compact ventricular cardiomyocytes from human pluripotent stem cells. Nat. Commun. 12, 3155 (2021).
Devalla, H. D. et al. Atrial-like cardiomyocytes from human pluripotent stem cells are a robust preclinical model for assessing atrial-selective pharmacology. EMBO Mol. Med. 7, 394–410 (2015).
Pradhan, A. et al. FGF signaling enforces cardiac chamber identity in the developing ventricle. Development 144, 1328–1338 (2017).
Targoff, K. L. et al. Nkx genes are essential for maintenance of ventricular identity. Development 140, 4203–4213 (2013).
Bao, Z. Z., Bruneau, B. G., Seidman, J. G., Seidman, C. E. & Cepko, C. L. Regulation of chamber-specific gene expression in the developing heart by Irx4. Science 283, 1161–1164 (1999).
Bruneau, B. G. et al. Cardiomyopathy in Irx4-deficient mice is preceded by abnormal ventricular gene expression. Mol. Cell. Biol. 21, 1730–1736 (2001).
Koibuchi, N. & Chin, M. T. CHF1/Hey2 plays a pivotal role in left ventricular maturation through suppression of ectopic atrial gene expression. Circ. Res. 100, 850–855 (2007).
Xin, M. et al. Essential roles of the bHLH transcription factor Hrt2 in repression of atrial gene expression and maintenance of postnatal cardiac function. Proc. Natl. Acad. Sci. USA. 104, 7975–7980 (2007).
Wu, S.-P. et al. Atrial identity is determined by a COUP-TFII regulatory network. Dev. Cell 25, 417–426 (2013).
Niederreither, K. et al. Embryonic retinoic acid synthesis is essential for heart morphogenesis in the mouse. Development 128, 1019–1031 (2001).
Lee, J. H., Protze, S. I., Laksman, Z., Backx, P. H. & Keller, G. M. Human pluripotent stem cell-derived atrial and ventricular cardiomyocytes develop from distinct mesoderm populations. Cell Stem Cell 21, 179–194 (2017).
Bruneau, B. G. et al. A murine model of Holt–Oram syndrome defines roles of the T-box transcription factor Tbx5 in cardiogenesis and disease. Cell 106, 709–721 (2001).
van Ouwerkerk, A. F. et al. Patient-specific TBX5-G125R variant induces profound transcriptional deregulation and atrial dysfunction. Circulation 145, 606–619 (2022).
Nadadur, R. D. et al. Pitx2modulates a Tbx5-dependent gene regulatory network to maintain atrial rhythm. Sci. Transl. Med. 8, 354ra115 (2016).
Dai, W. et al. A calcium transport mechanism for atrial fibrillation in Tbx5-mutant mice. eLife 8, e41814 (2019).
Laforest, B. et al. Atrial fibrillation risk loci interact to modulate Ca2+-dependent atrial rhythm homeostasis. J. Clin. Invest. 129, 4937–4950 (2019).
Kathiriya, I. S. et al. Modeling human TBX5 haploinsufficiency predicts regulatory networks for congenital heart disease. Dev. Cell 56, 292–309 (2021).
Steimle, J. D. & Moskowitz, I. P. TBX5: a key regulator of heart development. In Current Topics in Developmental Biology (ed Frasch, M.) Vol. 122, 195–221 (Academic Press, 2017).
Ni, L. et al. Atrial-specific gene delivery using an adeno-associated viral vector. Circ. Res. 124, 256–262 (2019).
Hulsurkar, M. M. et al. Atrial-specific LKB1 knockdown represents a novel mouse model of atrial cardiomyopathy with spontaneous atrial fibrillation. Circulation 144, 909–912 (2021).
Horsthuis, T. et al. Distinct regulation of developmental and heart disease-induced atrial natriuretic factor expression by two separate distal sequences. Circ. Res. 102, 849–859 (2008).
Lu, F. et al. CMYA5 establishes cardiac dyad architecture and positioning. Nat. Commun. 13, 2185 (2022).
Takeuchi, J. K. et al. Chromatin remodelling complex dosage modulates transcription factor function in heart development. Nat. Commun. 2, 187 (2011).
Georges, R., Nemer, G., Morin, M., Lefebvre, C. & Nemer, M. Distinct expression and function of alternatively spliced Tbx5 isoforms in cell growth and differentiation. Mol. Cell. Biol. 28, 4052–4067 (2008).
Stuart, T., Srivastava, A., Madad, S., Lareau, C. A. & Satija, R. Single-cell chromatin state analysis with Signac. Nat. Methods 18, 1333–1341 (2021).
Hao, Y. et al. Integrated analysis of multimodal single-cell data. Cell 184, 3573–3587 (2021).
Butler, A., Hoffman, P., Smibert, P., Papalexi, E. & Satija, R. Integrating single-cell transcriptomic data across different conditions, technologies, and species. Nat. Biotechnol. 36, 411–420 (2018).
Trapnell, C. et al. The dynamics and regulators of cell fate decisions are revealed by pseudotemporal ordering of single cells. Nat. Biotechnol. 32, 381–386 (2014).
Yang, X. H. et al. Transcription-factor-dependent enhancer transcription defines a gene regulatory network for cardiac rhythm. Elife 6, e31683 (2017).
Arnolds, D. E. et al. TBX5 drives Scn5aexpression to regulate cardiac conduction system function. J. Clin. Invest. 122, 2509–2518 (2012).
van Gorp, P. R. R. et al. Sbk2, a newly discovered atrium-enriched regulator of sarcomere integrity. Circ. Res. 131, 24–41 (2022).
Ackerman, M. J. & Mohler, P. J. Defining a new paradigm for human arrhythmia syndromes: phenotypic manifestations of gene mutations in ion channel- and transporter-associated proteins. Circ. Res. 107, 457–465 (2010).
Bosada, F. M. et al. An atrial fibrillation-associated regulatory region modulates cardiac Tbx5 levels and arrhythmia susceptibility. eLife 12, e80317 (2023).
Hnisz, D. et al. Super-enhancers in the control of cell identity and disease. Cell 155, 934–947 (2013).
Leblanc, F. J. A. et al. Transcriptomic profiling of canine atrial fibrillation models after one week of sustained arrhythmia. Circ. Arrhythm. Electrophysiol. 14, e009887 (2021).
Ma, S. et al. Chromatin potential identified by shared single-cell profiling of RNA and chromatin. Cell 183, 1103–1116 (2020).
Nakajima, H. et al. Atrial but not ventricular fibrosis in mice expressing a mutant transforming growth factor-β1 transgene in the heart. Circ. Res. 86, 571–579 (2000).
Cui, M. et al. Dynamic transcriptional responses to injury of regenerative and non-regenerative cardiomyocytes revealed by single-nucleus RNA sequencing. Dev. Cell 53, 102–116 (2020).
Roselli, C. et al. Multi-ethnic genome-wide association study for atrial fibrillation. Nat. Genet. 50, 1225–1233 (2018).
Schoenfelder, S. et al. The pluripotent regulatory circuitry connecting promoters to their long-range interacting elements. Genome Res. 25, 582–597 (2015).
Mumbach, M. R. et al. HiChIP: efficient and sensitive analysis of protein-directed genome architecture. Nat. Methods 13, 919–922 (2016).
Servant, N. et al. HiC-Pro: an optimized and flexible pipeline for Hi-C data processing. Genome Biol. 16, 259 (2015).
Lareau, C. A. & Aryee, M. J. hichipper: a preprocessing pipeline for calling DNA loops from HiChIP data. Nat. Methods 15, 155–156 (2018).
Durand, N. C. et al. Juicer provides a one-click system for analyzing loop-resolution Hi-C experiments. Cell Syst 3, 95–98 (2016).
Man, J. C. K. et al. Genetic dissection of a super enhancer controlling the Nppa-Nppbcluster in the heart. Circ. Res. 128, 115–129 (2021).
Chen, H. et al. BMP10 is essential for maintaining cardiac growth during murine cardiogenesis. Development 131, 2219–2231 (2004).
Xia, B. et al. Machine learning uncovers cell identity regulator by histone code. Nat. Commun. 11, 2696 (2020).
Shim, W. J. et al. Conserved epigenetic regulatory logic infers genes governing cell identity. Cell Syst 11, 625–639 (2020).
Zhou, P. et al. Dynamic changes in P300 enhancers and enhancer-promoter contacts control mouse cardiomyocyte maturation. Dev. Cell 58, 898–914 (2023).
Yuan, S., Norgard, R. J. & Stanger, B. Z. Cellular plasticity in cancer. Cancer Discov. 9, 837–851 (2019).
LeBleu, V. S. & Neilson, E. G. Origin and functional heterogeneity of fibroblasts. FASEB J. 34, 3519–3536 (2020).
Lickert, H. et al. Baf60c is essential for function of BAF chromatin remodelling complexes in heart development. Nature 432, 107–112 (2004).
Robbe, Z. L. et al. CHD4 is recruited by GATA4 and NKX2-5 to repress noncardiac gene programs in the developing heart. Genes Dev. 36, 468–482 (2022).
Panigrahi, A. & O’Malley, B. W. Mechanisms of enhancer action: the known and the unknown. Genome Biol. 22, 108 (2021).
Sinner, M. F. et al. Integrating genetic, transcriptional, and functional analyses to identify 5 novel genes for atrial fibrillation. Circulation 130, 1225–1235 (2014).
Fu, J.-D. D. et al. Direct reprogramming of human fibroblasts toward a cardiomyocyte-like state. Stem Cell Rep. 1, 235–247 (2013).
Holm, H. et al. Several common variants modulate heart rate, PR interval and QRS duration. Nat. Genet. 42, 117–122 (2010).
Hoesl, E. et al. Tamoxifen-inducible gene deletion in the cardiac conduction system. J. Mol. Cell. Cardiol. 45, 62–69 (2008).
Muzumdar, M. D., Tasic, B., Miyamichi, K., Li, L. & Luo, L. A global double-fluorescent Cre reporter mouse. Genesis 45, 593–605 (2007).
Peron, S. P., Freeman, J., Iyer, V., Guo, C. & Svoboda, K. A cellular resolution map of barrel cortex activity during tactile behavior. Neuron 86, 783–799 (2015).
Agah, R. et al. Gene recombination in postmitotic cells. Targeted expression of Cre recombinase provokes cardiac-restricted, site-specific rearrangement in adult ventricular muscle in vivo. J. Clin. Invest. 100, 169–179 (1997).
Wang, S., Guo, Y. & Pu, W. T. AAV gene transfer to the heart. Methods Mol. Biol. 2158, 269–280 (2021).
Jiang, J., Wakimoto, H., Seidman, J. G. & Seidman, C. E. Allele-specific silencing of mutant Myh6transcripts in mice suppresses hypertrophic cardiomyopathy. Science 342, 111–114 (2013).
Nadelmann, E. R. et al. Isolation of nuclei from mammalian cells and tissues for single-nucleus molecular profiling. Curr Protoc 1, e132 (2021).
McGinnis, C. S., Murrow, L. M. & Gartner, Z. J. DoubletFinder: doublet detection in single-cell RNA sequencing data using artificial nearest neighbors. Cell Syst. 8, 329–337(2019).
Hafemeister, C. & Satija, R. Normalization and variance stabilization of single-cell RNA-seq data using regularized negative binomial regression. Genome Biol. 20, 296 (2019).
Subramanian, A. et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. USA. 102, 15545–15550 (2005).
Chen, E. Y. et al. Enrichr: interactive and collaborative HTML5 gene list enrichment analysis tool. BMC Bioinformatics 14, 128 (2013).
McLean, C. Y. et al. GREAT improves functional interpretation of cis-regulatory regions. Nat. Biotechnol. 28, 495–501 (2010).
Dobin, A. et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29, 15–21 (2013).
Cui, M. & Olson, E. N. Protocol for single-nucleus transcriptomics of diploid and tetraploid cardiomyocytes in murine hearts. STAR Protoc 1, 100049 (2020).
Phanstiel, D. H., Boyle, A. P., Heidari, N. & Snyder, M. P. Mango: a bias-correcting ChIA-PET analysis pipeline. Bioinformatics 31, 3092–3098 (2015).
Lareau, C. A. & Aryee, M. J. diffloop: a computational framework for identifying and analyzing differential DNA loops from sequencing data. Bioinformatics 34, 672–674 (2018).
Li, H. & Durbin, R. Fast and accurate short read alignment with Burrows–Wheeler transform. Bioinformatics 25, 1754–1760 (2009).
Song, F., Xu, J., Dixon, J. & Yue, F. Analysis of Hi-C data for discovery of structural variations in cancer. In Hi-C Data Analysis: Methods and Protocols (eds Bicciato, S. & Ferrari, F.) 143–161 (Springer, 2022).
Durand, N. C. et al. Juicebox provides a visualization system for Hi-C contact maps with unlimited zoom. Cell Syst. 3, 99–101 (2016).
Chen, K. et al. DANPOS: dynamic analysis of nucleosome position and occupancy by sequencing. Genome Res. 23, 341–351 (2013).
Acknowledgements
M.E.S. and M.T. were supported by T32HL007572 and F32 F32HL163877. W.T.P. was supported by R01HL156503. Y.C., F.L. and P.W. were supported by AHA Postdoctoral Fellowships. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
M.E.S. and Y.C. contributed equally to this study. M.E.S. and Y.C. conceived of the study; designed and performed the experiments; and analyzed the data. X.Z., C.P.-C. and K.A. contributed to the data analysis. O.B.-T., B.N.A., Q.M., E.K., H.W., J.M.G., L.H., M.K.S., M.A.T., P.W., F.L., M.G., M.P. and R.H.B. contributed data, reagents and analyses. V.B., K.C., J.G.S., C.E.S., I.P.M. and W.T.P. oversaw the project and provided resources. M.E.S. and W.T.P. wrote the manuscript, with input from Y.C. and the other authors.
Corresponding author
Ethics declarations
Competing interests
The authors have no competing interests to declare.
Peer review
Peer review information
Nature Cardiovascular Research thanks Vincent Christoffels and the other, anonymous, reviewer(s), for their contribution to the peer review of this work. Primary Handling Editor: Vesna Todorovic, in collaboration with the Nature Cardiovascular Research team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended Data
Extended Data Fig. 1 Characterization of the cardiac expression domain of AAV9:Nppa-EGFP.
a-b, Hcn4CreERT2/+; Rosa26LSL-Tomato pups were injected with 2 X 1011 viral genomes per gram bodyweight (VG/g) AAV9:Nppa-EGFP at P8 and injected with tamoxifen at P21 and P22 to activate tomato expression in the conduction system including the sinoatrial and atrioventricular nodes. Hearts were harvested in PBS and brightfield, GFP, and Tomato fluorescent images were acquired. Mutually exclusive GFP and tomato signals were observed in the atrium and the SA node, respectively. A similar result was observed with administration at P2. c, Hearts were sectioned and stained with WGA. Tomato signal marked the sinoatrial node (SAN) and atrioventricular node (AVN) but not the working myocardium. GFP was restricted to the working myocardium. A similar staining pattern was observed in 4 other littermates.
Extended Data Fig. 2 Phenotypic characterization of Tbx5AKO mice.
a, Trichrome staining of Tbx5Flox/Flox and Tbx5AKO hearts. b, RT-qPCR quantification of Postn (periostin) and Col1a1 (collagen type 1 alpha 1 chain), two indicators of fibrosis (n = 3 control and 4 KO mice). Unpaired two-sided t-test. Error bars represent mean values +/-SEM. c-d, Immunostaining for sarcomeric α-actinin (SAA) or FSD2, markers of the Z line and the junctional Sarcoplasmic reticulum, respectively, in the left atrium of the indicated genotypes. Localization of both proteins is disrupted in Tbx5AKO atria. e, Pattern of SAA signal intensity. SAA intensity along the long axis of cardiomyocytes demonstrated a periodic signal in control, consistent with regular position of sarcomere Z-lines, and loss of periodicity in Tbx5AKO. f, Preserved ventricular function of Tbx5AKO mice. Tbx5Flox/Flox mice were treated with AAV:Nppa-EGFP (control) or AAV:Nppa-Cre (Tbx5AKO) at P2. Echocardiography was performed at P20. LVID;d, left ventricular internal diameter at end diastole. EF, ejection fraction. n = 5 control and 4 KO mice. Graphs show mean ± SEM. g, Surface EKG recordings. Orange and black bars highlight successive RR intervals. h, Poincaré plots. The RR interval of greater than 1500 beats on EKG recordings is plotted versus the RR interval of the subsequent beat (RR[ + 1]). to visualize the dispersion of interbeat intervals in Tbx5AKO, consistent with atrial fibrillation. i, Standard deviation of the RR interval, a measure of heart rate irregularity, was calculated for at least 1500 beats for each group at P21. n = 4 control and 9 KO mice. Unpaired two-sided t-test: ***, P = 0.005. Graphs show mean ± SEM. j, Time course of heart rate irregularity. Serial EKGs were acquired from control or Tbx5AKO mice at the indicated time points. SDRR was measured and compared between groups at each time point using a two-way ANOVA with Sidek’s multiple comparison test. **, P < 0.01. ***, P < 0.001. For P14 and P17 timepoints, n = 3 control and 3 KO mice. For P8 and P21 timepoints, n = 5 control and 8 KO mice. Graphs show mean ± SEM. k, Simultaneous intracardiac and surface ECG recordings demonstrate normal synchronous atrial-ventricular rhythm in Tbx5Flox/+ mice injected with AAV-Nppa-Cre. l, In contrast, animals with complete atrial ablation of Tbx5 (AAV-Nppa-Cre + Tbx5flox/flox) demonstratee nearly continuous low-amplitude atrial activity and irregularly irregular ventricular response consistent with atrial fibrillation.
Extended Data Fig. 3 Tbx5 overexpression atrializes ventricular myocytes.
a, Strategy to generate TBX5-OE ventricles. A full length Tbx5 cDNA downstream of the ubiquitous CAG promoter was activated by the cardiomyocyte specific Myh6-Cre transgene. b-c, Volcano plot comparing the change in gene expression of mouse left ventricle overexpressing Tbx5 compared to control LV. aCM genes are marked in red in (a) and vCM genes are denoted in blue in (b). b-c, Wald’s test followed by Benjamini Hochberg correction. d-e, Fisher’s exact test (two tailed) was performed to determine if changes in chamber selective gene expression downstream of Tbx5 overexpression were significant. f-h, Staining for MYL4, MYL7, and MYL2 in atria and ventricles of control and TBX5-OE hearts. Staining for each marker was performed using 3 control hearts and 2 TBX5-OE hearts, and representative images are shown.
Extended Data Fig. 4 Single cell dataset metrics.
a, Parameters from single nucleus datasets. Mean values per nucleus: nCount_ATAC, number of ATAC fragments; nFeature_ATAC, number of ATAC peaks with at least one read; nCount_RNA, number of RNA fragments; and nFeature_RNA, number of genes. b, Transcription start site (TSS) aggregation plots for the scATAC multiome datasets showing the expected enrichment of ATAC fragments. c, WNN UMAP of the dataset split by original sample. Each of the KO and control replicates have a high degree of overlap, demonstrating high reproducibility of cell state changes in Tbx5AKO atria.
Extended Data Fig. 5 Differentially expressed genes between myocyte clusters.
a, Volcano plot of differentially expressed genes (DEGs) between early and late pseudotime clusters in the control trajectory (Myocyte_4 vs. Myocyte_1, left) and the KO trajectory (Myocyte_5 vs. Myocyte_6; right). Wilcoxon rank sum test, Bonferroni correction. b. MA plot of RNA-seq experiment comparing P0 and P28 aCMs. c-d. Volcano plots shown in (a) overlayed with genes enriched in P28 and P0 aCMs. Wilcoxon rank sum test, Bonferroni correction. e. The proportion of DEGs from comparisons in (a) that overlap genes selectively expressed in aCMs at P0 and P28. We did not observe enrichment of P0 or P28 selective aCM genes in early or late pseudotime clusters, respectively. This suggested that pseudotime trajectories did not correspond to chronological time. f-g. GO biological process terms enriched for DEGs for the comparisons shown in (a). The top 10 terms enriched by genes upregulated in the indicated cluster are shown. Functional terms related to cardiomyocyte function were enriched in the late pseudotime clusters, Myo_1 (control) and Myo_6 (AKO). Two-tailed Fisher’s exact test, Bonferroni correction. h, WNN UMAP plot colored by a ‘functional cardiac gene’ index, which was calculated based on the aggregate expression of the six indicated genes, which are required for the efficient pumping function of aCMs.
Extended Data Fig. 6 A comparison of TBX5 RNA-seq datasets.
We compared snRNAseq data from Tbx5AKO aCMs to three other bulk RNA-seq datasets involving Tbx5 gain- or loss- of function. Control_aCM indicates Myocyte_1 and KO_aCM indicates Myocyte_6. a, c, e: Scatter plot of fold-change in Tbx5AKO aCMs compared to the other three datasets. Left, points are colored by significance in each dataset (Padj < 0.05). Right, genes with significant differential expression in both datasets are colored by chamber selectivity. Pie charts summarize the proportion of each class of genes within each quadrant. b, d, f: Enrichment of aCM or vCM genes in the indicated quadrants of the scatter plots. Fisher’s exact test, two-tailed. a-b. Comparison to LA tissue with ubiquitous, adult-induced inactivation of Tbx5 (Tbx5iKO; Nadadur et al., 2016). c-d. Comparison to LA tissue with mild Tbx5 upregulation due to deletion of an intronic regulatory element (Tbx5Re(int)KO; Bosada et al., 2023). e-f. Comparison to LV tissue with Tbx5 overexpression in cardiomyocytes (Tbx5-OE; this study). g. Comparison of changes in expression of aCM- and vCM-selective genes across all four datasets. Fold-change was calculated between condition with higher Tbx5 (numerator) to condition with lower Tbx5 (denominator). Genes were ordered by ascending Log2(WT/TBX5iKO). Genes not detected (ND) for a given experiment are colored white. Normalized enrichment score (NES) and false discovery rate (FDR) values from GSEA using the aCM-selective or vCM-selective gene lists are shown. Negative NES indicates enrichment in the genes upregulated in the lower Tbx5 condition, whereas positive NES indicates enrichment in the genes upregulated in the higher Tbx5 condition.
Extended Data Fig. 7 Characterization of myocyte differentially accessible regions.
Heatmap (a) shows the patterns of differential accessibility between myocyte clusters. The rows contain the union of regions with differential accessibility in the four pairwise comparisons shown in Fig. 5a. ATAC signal in each region is shown for myocyte clusters 1 (control_aCM cluster), 4, 6 (KO_aCM cluster), and 5. The regions are grouped (groups a-f) by their pattern of accessibility change in the four pairwise comparisons. Please also refer to Supp. Table 4. Arrows denote significant enrichment in one cluster compared to another. These six groups fit two predominant patterns: those with and those without predominant accessibility in the control aCM cluster (Myocyte_1). Most regions with predominant accessibility in the control aCM cluster were occupied by TBX5 and had GO terms related to cardiac cell development or striated muscle contraction (b). In contrast, a minority of regions without predominant accessibility in the control aCM cluster were occupied by TBX5 and had GO terms that were atypical for cardiomyocytes (c). Groups with less than 200 regions are not shown in the heatmap.
Extended Data Fig. 8 Proximity and predicted gene linkages demonstrate the regulation of the atrial GRN by control peaks.
a, The different types of association of a genomic region with a gene: (1) Linkage (L). Region-to-gene linkages are predicted based on co-variance of accessibility and expression on a nucleus-by-nucleus basis in the multiome data. (2) Proximity (P). Region-to-gene relationships are inferred by proximity of the region to the gene’s transcriptional start site. (3) Proximity and linkage (P + L). A region-to-gene association can be supported by both proximity and linkage. b, Genes were associated with control and KO regions by linkage (n = 2 control and 2 KO multiome biological replicates). Ratio of gene expression between the control and KO aCM clusters was plotted and compared between groups by the two-sided Mann-Whitney test. P = 9.1E-48. c, Association of control and KO ATAC regions with aCM and vCM genes. Associations were made based on proximity, linkage, or both. d, aCM and vCM genes were interrogated for associations with control and KO ATAC regions by proximity, linkage, or both.
Extended Data Fig. 9 Motif analysis of control and KO ATAC regions.
a, Aggregation plots for H3K27Ac at control and KO regions. b, Heatmaps of the H3K27Ac signal at control and KO peaks. c, Top-enriched motifs identified in control regions. The TBOX motif was the most enriched, followed by MEF2. An extended table of non-redundant motifs showing the top 21 most enriched motifs in control regions. d, Transcription factor footprinting analysis demonstrates footprints at TBOX and MEF2 motifs in control clusters (Myocyte_1 and Myocyte_4) compared to KO clusters (Myocyte_5 and Myocyte_6). e, Top-enriched motifs identified in KO regions and extended table of the top non-redundant motifs. f. Occupancy of control and KO ATAC regions by cardiac TFs in aCMs. Occupancy data is from GEO GSE2150653.
Extended Data Fig. 10 Chromatin loops link TBX5 dependent enhancers with atrial genes.
a, Contact maps of Myl7 or Bmp10. Black boxed regions are loops called in each sample and blue boxed regions mark differential loops that are significantly stronger in control samples. b, Genes near control anchors grouped by adjacency to aCM-selective, vCM-selective, or non-chamber selective expression. Control anchors were present near 62 aCM-selective genes and of these, 46 were expressed at greater levels in control Myocyte_1 compared to KO Myocyte_6. Notable genes from previous figures include Nppa, Bmp10, Sbk2 and Myl7. 38 non-chamber selective genes were also upregulated in control samples and linked to enhancers by TBX5-dependent looping. These included Gja1 and Tead1. Only 5 vCM-selective genes were found near control anchors. c, 50 genes neighbored KO anchors. These genes included 5 aCM-selective genes and 3 vCM-selective genes. Most of the differentially expressed genes near KO anchors were more highly expressed in Myocyte_6 (KO) compared to Myocyte_1 (Ctrl).
Supplementary information
Supplementary Tables
Supplementary Tables 1–5 and 7.
Supplementary Table
diffloop HiChIP results.
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 1
Uncropped western gels.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 5
Statistical source data.
Source Data Fig. 7
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sweat, M.E., Cao, Y., Zhang, X. et al. Tbx5 maintains atrial identity in postnatal cardiomyocytes by regulating an atrial-specific enhancer network. Nat Cardiovasc Res 2, 881–898 (2023). https://doi.org/10.1038/s44161-023-00334-7
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s44161-023-00334-7
This article is cited by
-
Cardiac epigenome in heart development and disease
Nature Reviews Cardiology (2026)
-
Single-nuclei multiomics analysis identifies abnormal cardiomyocytes in a murine model of cardiac development
Nature Communications (2025)
-
PITX2 dosage-dependent changes in pacemaker cell state underlie sinus node dysfunction and atrial arrhythmias
Nature Communications (2025)
-
Genetic and molecular underpinnings of atrial fibrillation
npj Cardiovascular Health (2024)