Abstract
Obesity and depression are likely to co-occur. However, there are few reports on the relationship between obesity and depression. We aimed to investigate the effect of high-fat diet combined with chronic restraint stress on depressive-like behaviors, focusing on the phenotypic transformation of astrocytes. Male C57BL/6 mice were randomly divided into four equal groups: control, high-fat diet (HFD), chronic restraint stress (CRS) and HFD + CRS groups. They were subjected to an 8-week high-fat diet and 3-week restraint stress stimulation. In vitro, palmitic acid (PA) and corticosterone (Cort) were used to mimic HFD and CRS respectively on C8-D1A astrocytes. Our results showed that HFD aggravates anxiety and depression-like behaviors and learning and memory deficits induced by CRS, as reflected by sucrose preference, forced swimming test, tail suspension tests, open field test and the Morris water maze. The expression level of C3 protein in the hippocampus of the mice in the HFD + CRS group was three times that of the CON group. HFD combined with CRS significantly inhibited the protein expression of the Wnt/β-catenin signaling pathway. Consistent with the results of animal experiments, the results of the in vitro experiments showed that the protein expressions of A1 astrocytes marker in C8-D1A astrocytes were much higher in the PA + Cort group. And the protein expressions the Wnt/β-catenin signaling pathway-associated proteins were obviously lower in the PA + Cort group. Furthermore, Wnt/β-catenin pathway agonist SKL2001 treatment decreased the A1 astrocytes marker expressions in C8-D1A astrocytes, and improves the anxiety and depression-like behaviors and learning and memory deficits in HFD mice combined with CRS. This study suggested that HFD combined with CRS could promote the transformation of astrocytes into A1 type and the Wnt/β-catenin signaling pathway may be involved in this process.
Similar content being viewed by others
Introduction
Depression is one of the most common mental illnesses, manifesting mainly as anhedonia, low mood and cognitive impairment1. Recently, clinical and epidemiological data suggest that obesity and depression tend to co-occur within individuals2. The incidence of obesity has increased dramatically across all age groups worldwide, and obesity has been shown to correlate with an increased risk of mental disorders such as depression3. Epidemiological evidence showed that obesity and depression exhibit a bidirectional causal relationship4. Previous studies have identified potential mechanisms, including neuroinflammation, metabolic dysfunction, and gut-brain axis abnormalities, which may play a significant role in this relationship5,6,7. However, links that explain the relationship between obesity and depression remains obscure.
Feeding of a high-fat-diet (HFD) is commonly used to establish animal models of obesity. Meanwhile, the chronic restraint stress (CRS) model has been widely used as a highly predictive and valid animal model of depression8,9. In clinical practice, it is often observed that patients with depression are simultaneously exposed to high-fat diet and chronic stress. These two factors interact, jointly exacerbating depressive symptoms and disease progression10. Since a single predisposing factor can only mimic one or part of the pathogenesis of depression, exploring the pathogenesis of depression by combining high-fat diet and depression can better mimic the current situation of depression induced by environmental factors in modern society.
Studies have shown that astrocyte dysfunction is associated with the pathogenesis of depression11. Similarly, our previous study found that high-fat diet induced hippocampal astrocyte activation and increased anxiety and tension in mice12. It has been shown that reactive astrocytes (RAS) are extremely heterogeneous and can be classified into different subtypes such as A1 astrocytes (A1s) and type A2 astrocytes (A2s) based on the gene expression, A1s highly upregulate gene expression of the classical complement pathway, which has an injurious effect on the synapses and may be deleterious, while A2s upregulate the expression of neurotrophic factors that may be neuroprotective13. In the cerebral ischemia model, astrocytes in the early stage exhibit a unique reactive phenotype, displaying both inflammation-related characteristics of A1-type cells and certain neuroprotective properties of A2-type cells. This indicates that the functional state of astrocytes is not strictly confined to either A1 or A2 but is dynamically regulated by factors such as time, injury severity, and signals from the local microenvironment14. Clinical studies have shown that the level of C3 in the plasma of patients with depression is significantly higher than that in the control group15. Currently there are few reports on the correlation between A1/A2 phenotypic transformation of astrocytes and psychiatric disorders such as depression. However, the combined effect of HFD and CRS on the phenotype transformation of AS remains unknown.
Known for its role in intercellular communication and cell proliferation, the Wnt/β-catenin signaling has been implicated in diverse biological phenomena associated with depression, spanning neurodevelopmental to neurodegenerative processes16. Clinical studies have shown that the Wnt/β-catenin signaling pathway is a potential influencing factor in the pathogenesis of depression17. Besides, Wnt/β-catenin signaling was found to be associated with astrocyte differentiation in the experiments on ischemia-induced neuronal apoptosis18. Studies have shown that in ischemic stroke, the activation of the Wnt/β-catenin signaling pathway can lead to a decrease in C3 expression, thereby inhibiting the polarization of astrocytes into the A1s19. However, the relation between the phenotype transformation of AS and Wnt/β-catenin signaling pathway was rarely reported in depression.
We hypothesize that the combined effect of the HFD and CRS can inhibit the Wnt/β-catenin signaling pathway, thereby inducing the polarization of astrocytes into the neurotoxic A1 type and leading to depression-related behaviors. This study aims to elucidate the relationship between HFD + CRS and the transformation of astrocytes into A1s, and to investigate whether the Wnt/β-catenin signaling pathway serves as a therapeutic target for obesity-related depression by establishing in vivo and in vitro models. The neurobehavioral effects of HFD and CRS was evaluated using the Sucrose preference test (SPT), Forced swimming test (FST), Tail suspension test (TST), Open field test (OFT) and Morris water maze (MWM) test. The transformation of astrocytes was assessed by western blotting and immunohistochemistry in vivo and in vitro. The effect of Wnt/β-catenin signaling pathway on the phenotype transformation of AS was assessed using in vitro cultured cells. Finally, to confirm the role of AS phenotype transformation in anxiety and depression-like behaviors, pharmacological activation of Wnt/β-catenin signaling pathway were performed. The outcomes of our study provide a basis for further exploration to the mechanism of depression. Our study elucidated the correlation between the model of the HFD combined with CRS and the phenotypic transformation of astrocytes. Moreover, the Wnt/β-catenin signaling pathway plays a crucial role in the phenotypic transformation of astrocytes. This provides a new target for the treatment of patients with depression.
Results
Effects of HFD and CRS on the depression-like behaviors
As shown in Fig. 1A, after 8 weeks of the experiment, compared to the control group, the body weight of mice significantly increased in the HFD group, whereas it significantly decreased in the CRS group. Compared to the HFD group, the body weight of mice significantly decreased in the HFD + CRS group (Fig. 1B, Row Factor x Time: F (24, 189) = 9.446; Row Factor: F (8, 63) = 352.4; Time: F (2.856, 179.9) = 98.20;p < 0.05, η²=0.76). Before the experiment, there was no difference in the sucrose preference of mice among the groups. However, at the end of the experiment it was significantly decreased in the HFD and CRS groups as compared to the control group, and the decrease in the HFD + CRS group was more notably (Fig. 1D, F (3, 28) = 489.4, p < 0.05, η²=0.98). In addition, the total fluid intake (FIT) for SPT in four groups of mice was not different (Fig. 1C, F(4, 35) = 0.4379, p > 0.05, η²=0.02). FST and TST were used to measure depressive-like behaviors. In comparison to the control group, the FST and TST immobile time significantly increased in the HFD and CRS groups, while the HFD + CRS group had a higher immobile time compared to the HFD and CRS groups (Fig. 1E, F, F (3, 28) = 40.64, F (3, 28) = 60.53, p < 0.05, η²=0.81, η²=0.86, ). These results indicated that HFD could aggravate the depression-like behaviors induced by CRS in mice.
Effects of HFD and CRS on the anxiety and depression-like behaviors. (A) Schematic diagram of mice dealing with HFD and CRS. (B) Body weight of mice. (C) Total fluid intake test (FIT) for SPT. (D) The percentage of sucrose consumption in the SPT. (E) Immobility time was determined in the FST. (F) Immobility time was determined in the TST. Data were expressed as mean ± SEM (n = 8), *p < 0.05, **p < 0.01, ***p < 0.001 vs. control group; #p < 0.05, ##p < 0.01, ###p < 0.001 vs. CRS group; $p < 0.05, $$p < 0.01, $$$p < 0.001 vs. HFD group.
Effects of HFD and CRS on the anxiety behaviors
As shown in Fig. 2A, the locomotion trajectories revealed distinct exploration patterns among groups. The OFT results showed that either HFD or CRS did not influence the exercise capacity of mice (Fig. 2B, F(3, 28) = 0.6819, p > 0.05, η²=0.06), while compared to the control group, the HFD, CRS and HFD + CRS groups displayed a lower in ambulatory time in the center (Fig. 2C, F(3, 28) = 151.5, p < 0.05, η²= 0.94). These results indicated that HFD could aggravate the anxiety behaviors induced by CRS in mice.
Effects of HFD and CRS on the anxiety behaviors. (A) Representative locomotion trajectories of OFT. (B) Ambulatory distance in the OFT. (C) Time in the center zone in the OFT. Data were expressed as mean ± SEM (n = 8), *p < 0.05, **p < 0.01, ***p < 0.001 vs. control group; #p < 0.05, ##p < 0.01, ###p < 0.001 vs. CRS group; $p < 0.05, $$p < 0.01, $$$p < 0.001 vs. HFD group.
Effects of HFD and CRS on learning and memory function
Spatial memory and cognition abilities were assessed using the MWM. As training days increased, escape latency time decreased in in all groups. During the initial training (IT) phase, the mice in HFD and CRS groups found the platform for a more extended period than the control group. Furthermore, the escape latency in the HFD + CRS group was significantly higher than that of the HFD and CRS groups (Fig. 3A left). In the reverse training (RT) phase, the escape latency showed the same trend (Fig. 3A right, Row Factor x Time: F(18, 147) = 45.02; Row Factor: F(6, 49) = 706.2; Time: F(2.871, 140.7) = 601.7; p < 0.05, η²=0.64). The above results indicate that the mice in each group have a certain learning ability during the training process. However, the escape latency of the mice in the HFD + CRS group is significantly higher than that of the other groups, suggesting that there are obstacles in the learning process of the mice in the HFD + CRS group.
In the space exploration test during the space exploration test (SET) and reverse exploration test (RET) phases (Fig. 3C, F), the number of crossing the platform and the percentage of time spent in the target quadrant in the other 3 groups were significantly lower than that of the control group (Fig. 3D, G, F (3, 28) = 48.17, F (3, 28) = 23.19, p < 0.05, η²=0.83, η²= 0.71). Besides, mice in HFD + CRS group had a lower number of crossing frequencies and percentage of time spent in the target quadrant compared to the HFD or CRS group (Fig. 3E, H, F (3, 28) = 35.05, F (3, 28) = 28.91, p < 0.05, η²=0.78, η²=0.75 ). Additionally, no statistical difference in swimming speed was observed among the four groups throughout the test (Fig. 3B, Row Factor x Time: F(18, 147) = 0.5518; Row Factor: F(6, 49) = 2.146; Time: F(2.725, 133.5) = 1.037; p > 0.05, η²=0.06), which indicated that HFD or CRS does not change the spontaneous ability of the mice. These above results proved that HFD or CRS could impair the memory and cognition abilities of mice, and HFD combined with CRS could aggravate the memory and cognition abilities impairment.
HFD and CRS could induce learning and memory decline in mice. (A) Mean escape latency was determined daily in the IT and the RT stages. (B) Mean swimming speed in both the IT and RT stages. (C) Representative track images of SET (a single 60s probe trial was performed without the platform on day 6). (D) Number of platform area crossings in the SET stage. (E) Quadrant occupancy in the SET stage. (F) Representative track images of RET (a single 60s probe trial was performed without the platform on day 9). (G) Number of platform area crossings in the RET stage. (H) Quadrant occupancy in the RET stage. Data were expressed as mean ± SEM (n = 8), *p < 0.05, **p < 0.01, ***p < 0.001 vs. control group; #p < 0.05, ##p < 0.01, ###p < 0.001 vs. CRS group; $p < 0.05, $$p < 0.01, $$$p < 0.001 vs. HFD group.
HFD combined with CRS promoted the transformation of astrocytes into A1 type
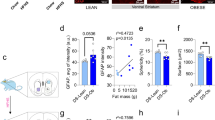
We tested the expression of astrocytes marker glial fibrillary acidic protein (GFAP), A1 astrocytes marker C3 and A2 astrocytes marker S100A10 to evaluate the phenotypic change of astrocytes. Immunofluorescence data showed that the fluorescence intensity of double labelled C3+ /GFAP+ (A1s) was considerably increased in the DG region of hippocampus of HFD + CRS group. While no significant change in the fluorescence intensity of the double-labelled S100A10+/ GFAP+ (A2s) was observed (Fig. 4A–D, p < 0.05). In addition, western blotting showed that the protein expressions of GFAP and C3 in HFD, CRS, and HFD + CRS groups were higher than in the control group. Compared to the HFD and CRS group, the protein expressions of GFAP and C3 in the HFD + CRS group were significantly higher (Fig. 4E–G, p < 0.05). However, it did not affect the protein expression of S100A10 (Fig. 4H, p > 0.05). These results suggested that HFD combined with CRS was associated with the transformation of astrocytes into A1 type.
Effects of HFD and CRS on the phenotypic change of astrocytes. (A–D) Representative confocal images labeled with GFAP (red), C3 (green) and S100A10 (green) in the DG region of hippocampus. Scale bar, 50 μm (× 20 magnifications). (E–H) Representative for western blotting photograms for GFAP, C3, and S100A10 in the mouse hippocampus of the control, HFD, CRS, and HFD + CRS groups. The expression values of GFAP (F), C3 (G), and S100A10 (H) were normalized with β-actin value. Data were presented as mean ± SEM (n = 3). *p < 0.05, **p < 0.01, ***p < 0.001 vs. control group; #p < 0.05, ##p < 0.01, ###p < 0.001 vs. CRS group; $p < 0.05, $$p < 0.01, $$$p < 0.001 vs. HFD group; n.s. not significant.
Correlation between C3 expression and behavioral deficits in HFD combined with CRS
The results of the correlation analysis showed that in the context of the combination of a high-fat diet and chronic restraint stress, the expression of C3 protein in the hippocampal tissue was negatively correlated with the sucrose preference and the time spent in the central area. It was positively correlated with the immobility time of the mice in the tail suspension test, the immobility time in the forced swimming test, and the escape latency (Fig. 5, p < 0.05).
Effects of PA and cort stimulation on the phenotypic change of C8-D1A astrocytes
Based on previous studies, C8-D1A cell line were selected, and palmitic (PA) and corticosterone (Cort) were used to mimic HFD and CRS respectively on C8-D1A astrocytes to explore the phenotypic transformation of AS20,21 (Fig. 6A). The optimal PA and Cort concentrations were identified using the CCK-8 kit. CCK-8 test showed that after PA or Cort treatment, the cell viability of C8-D1A astrocytes was significantly decreased dose-dependent, and approximately 50% of the cells remained viable at 100 µM exposure to 24 h (Fig. 6B, C). Based on these results, 100 µM PA, 100 µM Cort, and 100 µM PA + 100 µM Cort were used in further experiments. The results of double immunofluorescence verified that the immunofluorescence intensity of C3+/GFAP+ was notably elevated (Fig. 6D, E, p < 0.05), S100A10+/GFAP+ was unchanged in the PA, Cort, and PA + Cort groups (Fig. 6F, G, p > 0.05). In addition, the immunofluorescence intensity of C3+/GFAP+ increased most significantly in the PA + Cort group. Furthermore, western blotting showed that the protein expressions of GFAP and C3 in the PA and Cort groups were higher than that in the control group. The protein expressions of GFAP and C3 in the PA + Cort group were significantly higher than that in the PA and Cort groups(Fig. 6H–J p < 0.05). The protein expression of S100A10 had no statistical differences in the control, PA, Cort, and PA + Cort groups (Fig. 6K, p > 0.05). The findings above, in vivo and in vitro, suggested that HFD combined with CRS was associated with the transformation of astrocytes into A1 type, which may account for the animals’ poor performance in the behavior tests.
Effects of PA and Cort stimulation on the phenotypic change of C8-D1A astrocytes. (A) Schematic diagram showing treatment of C8-D1A astrocytes with PA and Cort. (B,C) Effect of Cort and PA on the viability of C8-D1A astrocytes. (D,E) C3+/GFAP+ expression in C8-D1A astrocytes detected by immunofluorescence, scale bar, 50 μm (× 40 magnifications). (F,G) S100A10+/GFAP+ expression in C8-D1A astrocytes detected by immunofluorescence, scale bar, 50 μm (× 40 magnifications). (H–K) Representative for western blotting photograms for GFAP, C3, and S100A10 in C8-D1A astrocytes of the control, PA, Cort, and PA + Cort groups. The expression values of GFAP (I), C3 (J), and S100A10 (K) were normalized with β-actin value. Data were presented as mean ± SEM (n = 3). *p < 0.05, **p < 0.01, ***p < 0.001 vs. control group; #p < 0.05, ##p < 0.01, ###p < 0.001 vs. Cort group; $p < 0.05, $$p < 0.01, $$$p < 0.001 vs. PA group; n.s. not significant.
Effect of HFD combined with CRS on the Wnt/β-catenin signaling pathway
To further explore the mechanism of the AS phenotypic transformation, we detected the expression of Wnt/β-catenin signaling pathway-associated proteins in mouse hippocampus. The protein expressions of Wnt3a, β-catenin, and p-GSK3β /GSK3β in HFD and CRS groups were lower than that of the control group, while these protein expressions in the HFD + CRS group were significantly lower compared to the HFD and CRS groups (Fig. 7A-D, p < 0.05). Immunofluorescence staining for Wnt3a showed that the fluorescence intensity was decreased in the HFD and CRS groups. In contrast, and the fluorescence intensity in HFD + CRS group was markedly lower compared to the HFD and CRS group (Fig. 7E–F, p < 0.05). These results indicated that HFD combined with CRS significantly inhibited the protein expression of the Wnt/β-catenin signaling pathway.
Effect of HFD combined with CRS on the Wnt/β-catenin signaling pathway. (A–D) Representative for western blotting photograms for Wnt3a, β-catenin, and p-GSK3β /GSK3β in the hippocampus of the control, HFD, CRS, and HFD + CRS groups. Wnt3a (B) and β-catenin (D) expression values were normalized with the β-actin value, respectively. The expression values of p-GSK3β (C) were normalized with GSK3β value. (E,F) Wnt3a (green) expression in the hippocampus was detected by immunofluorescence, Scale bar, 50 μm (× 20 magnifications). Data were presented as mean ± SEM (n = 3). *p < 0.05, **p < 0.01, ***p < 0.001 vs. control group; #p < 0.05, ##p < 0.01, ###p < 0.001 vs. CRS group; $p < 0.05, $$p < 0.01, $$$p < 0.001 vs. HFD group.
Effects of Wnt/β-catenin signaling pathway on the phenotype transformation of AS
To further study whether the Wnt/β-catenin signaling pathway is involved in the transformation of astrocytes, we treated C8-D1A astrocytes with the Wnt/β-catenin signaling agonist SKL2001. We segregated the C8-D1A astrocytes population into three groups: control, PA + Cort and PA + Cort + SKL2001 groups. The results showed that compared to the control group, the Wnt3a fluorescence intensity decreased in the PA + Cort group, while it was higher in the PA + Cort + SKL2001 group compared to the PA + Cort group (Fig. 8A-B, p < 0.05). Besides, we detected the translocation of β-catenin from the cytoplasm to the nucleus in C8-D1A by immunofluorescence staining, which is a typical hallmark of the activated Wnt/β-catenin signaling pathway. The immunofluorescence staining showed that nuclear β-catenin expression in the PA + Cort group decreased compared to the control group and increased in the PA + Cort + SKL2001 group compared to the PA + Cort group (Fig. 8C). Next, we detected the phenotype transformation of AS. Compared to the control group, the fluorescence intensity of double labelled C3+/GFAP+ (A1s) increased in the PA + Cort group, whereas it decreased in the PA + Cort + SKL2001 group (Fig. 8D, E, p < 0.05). However, the fluorescence intensity of the double-labelled S100A10+/ GFAP+ (A2s) did not differ between the three groups (Fig. 8F–G, p > 0.05). The protein expressions of GFAP, C3, and S100A10 showed the same trend through the three groups (Fig. 8H–J, p < 0.05; Fig. 8K, p > 0.05). Therefore, these results indicated that the activation of the Wnt/β-catenin signaling pathway could suppress the AS transformation to A1-like astrocyte.
Effects of Wnt/β-catenin signaling pathway on the phenotype transformation of AS. (A,B) Wnt3a expression in C8-D1A astrocytes detected by immunofluorescence. Bar, 50 μm (× 40 magnifications). (C) β-catenin expression in C8-D1A astrocytes detected by immunofluorescence, scale bar, 50 μm (× 60 magnifications). (D,E) C3+/GFAP+ expression in C8-D1A astrocytes detected by immunofluorescence, scale bar, 50 μm (× 40 magnifications). (F,G) S100A10+/GFAP+ and expression in C8-D1A astrocytes detected by immunofluorescence, scale bar, 50 μm (× 40 magnifications). (H–K) Representative for western blotting photograms for GFAP, C3, and S100A10 in C8-D1A astrocytes of the control, PA + Cort and PA + Cort + SKL2001 groups. The expression values of GFAP (I), C3 (J), and S100A10 (K) were normalized with β-actin value. Data were presented as mean ± SEM (n = 3). *p < 0.05, **p < 0.01, ***p < 0.001 vs. CON group; #p < 0.05, ##p < 0.01, ###p < 0.001 vs. PA + Cort group.
Reversal of anxiety and depression-like behaviors and learning and memory deficits by activating the Wnt/β-catenin signaling pathway
To determine whether activating Wnt/β-catenin signaling could rescue the anxiety and depression-like behaviors and improve the learning and memory deficits in mice, we utilized the SKL2001 agonist to activate Wnt signaling before the behavioral experiments. As expected, after treatment with SKL2001, compared to the HFD + CRS + vehicle group, sucrose preference increased significantly (Fig. 9B, F(4, 35) = 183.6, p < 0.05, η²=0.04), and the FST and TST immobile time significantly decreased in the HFD + CRS + SKL2001 group (Fig. 9C, D, F(4, 30) = 80.55; p < 0.05; F(4, 35) = 65.18; p < 0.05, η²=0.91, η²=0.88). In addition, the FIT for SPT in five groups of mice was not different (Fig. 9A, F(4, 35) = 0.4379, p > 0.05, η²=0.04). Moreover, the time in the center of HFD + CRS + SKL2001 group mice in the OFT was increased compared to the HFD + CRS + vehicle group (Fig. 9E, p < 0.05, η²= 0.94). In the MWM test, during the IT and RT phases, the mice in HFD + CRS + SKL2001 group found the platform for a shorter period than the HFD + CRS + vehicle group (Fig. 9F, Row Factor x Time: F(24, 196) = 33.40; Row Factor: F(6, 49) = 341.5; Time: F(2.493, 122.1) = 686.1; p < 0.05, η²=0.59). In the space exploration test, during the SET and RET phases, the number of crossing the platform and the percentage of time spent in the target quadrant in the HFD + CRS + SKL2001group was significantly higher than that of the HFD + CRS + vehicle group (Fig. 9G–J, F(4, 35) = 37.70; F(4, 35) = 88.27; F(4, 35) = 30.69; F(4, 35) = 100.8; p < 0.05, η²=0.81, η²=0.90, η²=0.77, η²=0.92). These findings suggest that the activation of Wnt/β-catenin pathway improved the anxiety and depression-like behaviors and learning and memory deficits caused by HFD combined with CRS.
Reversal of anxiety and depression-like behaviors and learning and memory deficits by activating the Wnt/β-catenin signaling pathway. (A) Total fluid intake test (FIT) for SPT. (B) The percentage of sucrose consumption in the SPT. (C) Immobility time was determined in the FST. (D) Immobility time was determined in the FST. (E)The time in the center zone in the OFT. (F) Mean escape latency was determined daily in the IT and the RT stages. (G) Number of platform area crossings in the SET stage. (H) Quadrant occupancy in the SET stage. (I) Number of platform area crossings in the RET stage. (J) Quadrant occupancy in the RET stage. Data were expressed as mean ± SEM (n = 8), *p < 0.05, **p < 0.01, ***p < 0.001 vs. control group; #p < 0.05, ##p < 0.01, ###p < 0.001 vs. CON + SKL2001 group. $p < 0.05, $$p < 0.01, $$$p < 0.001 vs. HFD + CRS + vehicle group.
Discussion
Obesity and depression are common chronic diseases, and the incidence of depression in obese people is higher than that in the general population22. Researches have shown that chronic restraint stress can lead to depression-like behaviors in mice23,24. Consumption of a high-fat diet (HFD) in mice could induce anxiety and depression25. However, the combined effect of HFD and CRS on the depression-like behaviors is rarely reported. The present study was designed to uncover the mechanism of depression-like behaviors in HFD-fed mice combined with chronic restraint stress. Our results showed that HFD combined with CRS could exacerbate anxiety symptoms and depressive-like behaviors, and impair spatial learning, short-term memory, and cognitive ability in mice. Interestingly, HFD combined with CRS promoted the transformation of astrocytes into A1 type along with the inhibition of Wnt/β-catenin signaling pathway. Furthermore, we observed that by activating the Wnt/β-catenin signaling pathway in HFD-fed mice combined with CRS, the transformation of astrocytes into A1 type could be suppressed and the anxiety and depression-like behaviors could be mitigated.
Researches have shown that astrocyte dysfunction is associated with the pathogenesis of depression26,27,28. Recent studies indicated that reactive astrocytes can be divided into two phenotypes: A1-like astrocytes (A1s) and A2-like astrocytes (A2s). A1s highly up-regulated gene expression in the classical complement pathway may be detrimental to synaptic damage, while A2s up-regulated neurotrophic factor expression, which may have a neuroprotective effect29,30. It has been reported that astrocytes could be transformed into A1-like reactive astrocytes in Alzheimer’s31. Multiple autopsy studies have revealed that a reduction in astrocyte density may be associated with depressive episodes32. Additionally, an elevation in the S100β protein of astrocytes in cerebrospinal fluid and serum might serve as a biomarker for depression33. Acute stress, such as lipopolysaccharide (LPS) and chronic social defeat stress (CSDS) stress, could induce the generation of A1 astrocytes in mice34,35, and inhibition of A1 differentiation of astrocytes could ameliorate depressive symptoms in mice36. In this study, we found that the HFD combined with CRS could promote the transformation of astrocytes into A1s in mice hippocampus. Besides, C8-D1A astrocytes were treated with PA and Cort to mimic HFD and CRS respectively in vitro. Consistent with the in vivo results, PA combined with Cort could significantly promote the transformation of astrocytes into A1s in C8-D1A astrocytes. Hence, in the present experiment, new insight that HFD combined with CRS was associated with the transformation of astrocytes into A1-like astrocytes may contribute to the animals’ poor performance in behavior tests. The hyperlipidemia induced by the HFD and the surge of glucocorticoids associated with CRS may synergistically impair the function of astrocytes, thus leading to the transformation of the astrocyte phenotype37. In response to different types of injuries or diseases, astrocytes may not simply switch between two fixed states but rather adopt a continuum of phenotypes38. In future research, we will conduct staged examinations on the high-fat diet combined with chronic restraint stress model. This will enable us to identify the diverse spectrum of astrocyte states and understand how they contribute to the diseases.
To investigate the underlying mechanism, we focused on Wnt/β-catenin signaling pathway. Previous study has reported that macrophages can directly induce the astrocyte phenotypic transformation through Wnt/β-catenin signaling pathway39. Wnt ligands, mainly secreted from astrocytes40 bind to receptor complexes on cytomembrane to stabilize and activate cytosolic β-catenin in cerebral ischemia41. In the Wnt/β-catenin signaling pathway, the loss of Wnt promotes the formation of destruction complexes that lead to cytosolic β-catenin degradation42, and activated Wnt3a could induce β-catenin translocating to nuclear43. Numerous studies have shown that in various models of nervous system diseases, the abnormality of the Wnt/β-catenin signaling pathway is closely related to the functional changes and phenotypic transformation of astrocytes44. These research results suggest that the Wnt/β-catenin signaling pathway plays a central role in the physiological and pathological processes of astrocytes. Therefore, we believe that giving priority to focusing on this pathway in this study is of great scientific significance.
Previous studies have indicated the essential roles of Wnt/β-catenin signaling in behaviors of depression45,46. In the population with depression, genes with differential expression in the Wnt/β-catenin signaling pathway tend to overlap with those related to depression47. Our data showed that HFD combined with CRS could inhibit the Wnt/β-catenin signaling pathway, and the translocation of β-catenin from the cytoplasm to the nucleus significantly decreased in C8-D1A astrocytes treated with PA in combination with Cort, which means the inhibition of Wnt/β-catenin signaling pathway. To further explore the role of the Wnt/β-catenin pathway in the astrocyte phenotypic transformation, the Wnt/β-catenin pathway agonist SKL2001 was used to activate the Wnt/β-catenin signaling pathway in HFD mice combined with CRS and C8-D1A astrocytes treated with PA in combination with Cort. As expected, the Wnt/β-catenin signaling pathway was dramatically activated while the astrocytes’ transformation into A1s was suppressed after SKL2001 administration, followed by the mitigation of the anxiety and depression-like behaviors and learning and memory deficits. Given the role of this pathway in astrocyte function and the observed astrocyte phenotype transformation in our animal model, targeting the Wnt/β - catenin pathway could potentially be a novel therapeutic approach. In our study, we applied HFD combined with CRS, which may cause HPA axis dysfunction. Excessive glucocorticoids may disrupt the normal homeostatic functions of astrocytes, such as glutamate uptake and neurotransmitter regulation, which could potentially contribute to the observed astrocyte phenotype changes48. In future research, we plan to measure HPA axis-related parameters, such as corticosterone levels, and investigate the interaction between HPA axis-related factors and the Wnt/β-catenin pathway in the context of astrocyte changes and behavioral outcomes.
Our study has several limitations. First, we did not perform a gender-difference analysis. Second, astrocyte-specific manipulations (e.g., conditional knockout or chemogenetic approaches) were not conducted to establish causal relationships. Without selectively targeting astrocyte function, it remains challenging to definitively confirm a direct causal link between the investigated factors and the observed astrocytic changes. Third, systemic metabolic markers were not measured. Given that obesity and high-fat diets profoundly influence systemic metabolism—and these metabolic alterations may reciprocally affect astrocyte function—the lack of such data constrains our mechanistic interpretation49. In future studies, we plan to quantify a comprehensive panel of metabolic biomarkers, including glucose, insulin, lipid profiles, and inflammatory cytokines, to elucidate the interplay between systemic metabolism and astrocyte pathology. Finally, we did not investigate alternative pathways regulating astrocyte function. Multiple signaling cascades, such as NF-κB, JAK/STAT, and oxidative stress pathways, are known to critically modulate astrocyte transformation50. Detailed exploration of these pathways in subsequent work will clarify the complex mechanistic network underlying astrocyte phenotypic shifts.
Conclusion
In conclusion, HFD combined with CRS aggravates anxiety and depression behaviors in association with the increased transformation of astrocytes to A1s. Furthermore, the Wnt/β-catenin signaling pathway may play a modulatory role in AS transformation to the A1-like astrocyte.
Materials and methods
Animals
Seventy two male C57BL/6 mice (age, 3–5 weeks; weight,13–16 g) were purchased from the China Institute for Food and Drug Control (111251231100256276). All experimental animals were operated in accordance with the provisions of the Experimental Animal Ethics Committee and reported in accordance with ARRIVE guidelines. The Animal Ethical and Welfare Committee of the North China University of Science and Technology approved animal husbandry and experiment procedures (2023-SY-038). Mice were housed in groups of 5 and maintained under 12-hour light-dark cycles at 20–24 °C, with ad libitum access to food and water.
Experimental procedure
Studies have demonstrated that the combination of the HFD and CRS exerts synergistic effects on dysregulating the HPA axis in rats, thereby exacerbating the progression of depression-like behaviors51. Experiment 1 (exp1): After one week of environmental adaptation, mice were then randomly divided into four groups: Control group (CON, n = 8), Chronic restraint stress group (CRS, n = 8), High-fat diet group (HFD, n = 8) and HFD + CRS group (n = 8). The control group mice and CRS group mice were fed a standard diet. The HFD group mice and HFD + CRS group mice were fed a diet containing 60% of calories derived from fat for 8 weeks52. Containing 60% of calories reflects people’s high-fat diet53. The CRS group mice and HFD + CRS group mice were performed by placing a mouse in a 50 ml tube and subjecting it to restraint for 6 h per day during the last three weeks54. Experiment 2 (exp2): Following adaptation to the housing conditions, mice were then randomly divided into three groups: Control group (CON, n = 8), HFD + CRS group (n = 8), Control + SKL2001 group (n = 8), HFD + CRS + vehicle group (n = 8) and HFD + CRS + SKL2001 group (n = 8). The Control and Control + SKL2001 groups mice were fed with standard diet, and the other three groups were fed with high-fat diet for 8 weeks. SKL2001 was dissolved in a 1:1 solution of ethanol: PBS to reach a final concentration of 0.5 mg/ml. The HFD + CRS + SKL2001 group mice received intraperitoneal injections of SKL2001 once every four days for 3 weeks in total55. The HFD + CRS + vehicle group mice received intraperitoneal injections of the 1:1 solution of ethanol: PBS once every four days for 3 weeks in total. All animals were euthanized under isoflurane anesthesia after behavioral experiments. All experimental procedures, such as the administration of the high - fat diet, chronic restraint stress induction, and behavioral testing, were scheduled at the same time of the day.
Cell culture and treatment
Procell Life Science & Technology Co.Ltd kindly provided C8-D1A (CL-0506). The C8-D1A cells were cultured in DMEM with 10% FBS and 1% streptomycin-penicillin and placed in an incubator at 5% CO2 and 37 °C. Palmitic acid (PA) was dissolved as a stock solution of 5 mM in 10% of bovine serum albumin (BSA) and diluted to concentrations of 50µM, 100µM, 200µM and 300µM in culture medium before use56. Corticosterone (Cort) was prepared in 1% dimethyl sulfoxide (DMSO) + 99% phosphate buffer solution (PBS) as a stock solution of 72.15 mM, which was diluted to concentration of 50µM, 100µM, 150µM, 200µM and 300µM with culture medium prior to use57. C8-D1A cells were incubated with 10 µl CCK-8 test solution in a 37 °C incubator for one hour. An enzyme marker detected the absorbance at 450 nm.
Behavioral experiments
At the end of modeling, the behavioral order was SPT, FST, TST, OFT, and MWM, and only one behavioral test was performed each day.
Sucrose preference test (SPT)
It is widely believed that the SPT is the best method of estimating depression based on anhedonia58. Before the test, mice had a 3-day adaptation to the 1% sucrose solution or water. After water deprivation for 24 h, each mouse was given free access to two bottles for 2 h. The water and sucrose water consumption were recorded. Sucrose preference was calculated as the sucrose preference % = [sucrose water intake (g)/total water intake(g)] × 100%.
Forced swimming test (FST)
Mice were placed individually in a cylinder (height 30 cm, diameter 20 cm) containing a depth of 15 cm water (20–22 °C). Water was changed every mouse. During the 6-min test, the mice were compelled into the water. The motilities of mice were recorded by an observer blinded to treatment conditions. Immobility was defined as the absence of escape-directed behaviors, except for minimal movements required to maintain the head above water. The total duration of immobile time (seconds) was recorded during the last 4 min of a single 6-min test session while initial 2 min was applied for mice adaption.
Tail suspension test (TST)
TST was used to evaluate susceptibility to depression-like behaviors59. Each mouse was suspended 30 cm above the table by the tail using paper adhesive tape for 6 min. We recorded each mouse immobility during the last 4 min. The total amount of time each mouse spent immobile (mice were considered immobile only when they hung passively without any movement of the head or paws. This protocol was rigorously adhered to by an investigator who was systematically blinded to the animals’ treatment conditions, ensuring an unbiased assessment of behavioral responses.
Open field test (OFT)
OFT served as a measure of anxiety-like behavior in this study60. Mice were adapted to the experimental room for one hour and were placed in the open field. The test was performed by recording the behavioral activity of mice for 10 min using the Activity Monitor software program (MED-associates, USA). The observation index including total distance and ambulatory time in the center zone. After the test of every mouse, the remaining odors were eliminated by cleaning the box with 75% alcohol to avoid affecting the results of the following mouse.
Morris water maze (MWM)
The Morris water maze (MWM) is a standard approach to assessing spatial memory and memory61.White dye was mixed with water in a circular pool. The circular pool was divided into four quadrants (N: north, S: south, E: east, W: west) and a white circular escape platform was submerged 1 cm in below the water in the center of the target quadrant. In the initial training stage (IT) and the reverse training stage (RT), each mouse was trained over 5 consecutive days with four trials, where the goal was to find the hidden platform. The four trials in a day are consecutive. The escape latency and swimming speed were tracked and recorded in IT and RT. In the space exploration test stage (SET) and the reverse exploration test stage (RET), a single 60s probe trial was performed without the platform on day 6 and day 9, and the following were recorded to evaluate reference memory: time spent in the target quadrant and the number of platform crossings. The mice analysis was performed using the Smart 3.0 system (Pan lab, Spain).
Western blotting
Mice were anesthetized by intraperitoneal injection of 0.2 ml 1% Pentobarbital Sodium following Guidelines for euthanasia of experimental animals. The hippocampal tissues or cells were placed in RIPA lysate (containing 1% PMSF and 1% protein phosphatase inhibitors) for 30 min. After centrifugation at 12,000 rotations per minute (rpm) for 10 min at 4 °C, the supernatant was collected, and the protein concentration was determined using the Nanodrop 2000. The protein in each sample was separated by 10% SDS-PAGE gel electrophoresis and followed by transferal to polyvinylidene fluoride (PVDF) membrane. Overnight incubation was carried out with the following primary antibodies: rabbit anti-Wnt3a (1:500, HUABIO), rabbit anti-β-catenin (1:2000, HUABIO), rabbit anti-GSK3β (1:1000, HUABIO), rabbit anti-p-GSK3β (1:1000, HUABIO), mouse anti-GFAP (1:1000, HUABIO), rabbit anti-S100A10 (1:2000, ABclonal), rabbit anti-C3 (1:1000, HUABIO), and rabbit anti-β-actin (1:100000, ABclonal). After overnight incubation, followed by three washes of 10 min TBST and then exposed to the secondary antibody (1:5000, Report) at room temperature for 2 h. In the final step, the signal was captured on chemiluminescence imaging system (CLINX-6300, China). Then, we used Image J software to measure the gray value of each band. Optical density analysis was performed using ImageJ and normalized by β-actin protein levels. Furthermore, phospho-protein signals were normalized to their total protein levels.
Immunofluorescence
In 5% BSA/PBS solution, the brain sections or cell-climbing slices were blocked for 1 h at room temperature and washed with PBS. Mouse anti-GFAP (1:100, HUABIO), rabbit anti-C3 (1:100, HUABIO), rabbit anti-S100A10 (1:100, ABclonal), rabbit anti-Wnt3a (1:100, HUABIO) and rabbit anti-β-catenin (1:100, HUABIO) were incubated with tissues overnight at 4 °C. Thereafter, the tissues were treated with secondary antibody (1:200, Protein-tech). The tissues nuclei were covered with the tablet containing DAPI. Finally, the sections were performed with an Olympus immunofluorescence microscope (Olympus, Tokyo, Japan; × 20 magnifications or × 40 magnifications). Quantitative analysis was conducted using ImageJ software as a measure of positive cells in the DG region of hippocampus and C8-D1A cells.
Statistical analyses
Values were presented as the means ± SEM. Prior to statistical analysis, normality of data (Shapiro-Wilk test) was checked. Data were analyzed using by one/two-way ANOVA, followed by post hoc Tukey-Kramer test to assess the differences between groups by SPSS 22. The MWM data in IT and RT were analyzed using a two-way repeated-measures analysis of variance (ANOVA), with day×group as the between-subjects factor and the measurement session as the within-subjects factor. Other data were evaluated by one-way ANOVA. For the correlation analysis of two continuous variables, Pearson’s correlation test was used, and a P < 0.05 was considered to indicate a statistically significant difference.
Data availability
Data availabilityThe datasets generated during and analyzed during the current study are available from the corresponding author on reasonable request.
References
Malhi, G. S., Mann, J. J. & Depression Lancet 392 (10161), 2299–2312 https://doi.org/10.1016/S0140-6736(18)31948-2 (2018).
Aslani, S. et al. The effect of high-fat diet on Rat’s mood, feeding behavior and response to stress. Transl. Psychiatr. 5 (11), e684 (2015).
Ji, T. et al. Diet change improves obesity and lipid deposition in high-fat diet-induced mice. Nutrients 15 (23), 4978 (2023).
Tyrrell, J. et al. Using genetics to understand the causal influence of higher BMI on depression. Int. J. Epidemiol. 48 (3), 834–848. https://doi.org/10.1093/ije/dyy223 (2019).
Ly, M. et al. Neuroinflammation: A modifiable pathway linking obesity, Alzheimer’s disease, and depression. Am. J. Geriatr. Psychiatr. 31 (10), 853–866. https://doi.org/10.1016/j.jagp.2023.06.001 (2023).
Haleem, D. J. Drug targets for obesity and depression: From serotonin to leptin. Curr. Drug Targ. 17 (11), 1282–1291 https://doi.org/10.2174/1389450117666151209123049 (2016).
Sun, Y., Zhu, H., Cheng, R., Tang, Z. & Zhang, M. Outer membrane protein Amuc_1100 of akkermansia muciniphila alleviates antibiotic-induced anxiety and depression-like behavior in mice. Physiol. Behav. 258, 114023. https://doi.org/10.1016/j.physbeh.2022.114023 (2023).
Park, M. J., Seo, B. A., Lee, B., Shin, H. S. & Kang, M. G. Stress-induced changes in social dominance are scaled by AMPA-type glutamate receptor phosphorylation in the medial prefrontal cortex. Sci. Rep. 8 (1), 15008 (2018).
Leng, L. et al. Menin deficiency leads to depressive-like behaviors in mice by modulating astrocyte-mediated neuroinflammation. Neuron 100 (3), 551–563e7 (2018).
da Silva, L. O. et al. Maternal exposure to high-fat diet modifies anxiety-like/depression-like behaviors and compounds of serotonergic system in offspring: A preclinical systematic review. Int. J. Dev. Neurosci. 81 (5), 371–385. https://doi.org/10.1002/jdn.10110 (2021).
Wu, L. et al. The role of regulatory T cells on the activation of astrocytes in the brain of high-fat diet mice following lead exposure. Chem. Biol. Interact. 351, 109740 (2022).
Li, K., Li, J., Zheng, J. & Qin, S. Reactive astrocytes in neurodegenerative diseases. Aging Dis. 10 (3), 664–675 (2019).
Thion, M. S., Ginhoux, F. & Garel, S. Microglia and early brain development: an intimate journey. Science 362 (6411), 185–189 (2018).
Lu, W. & Wen, J. Metabolic reprogramming and astrocytes polarization following ischemic stroke. Free Radic Biol. Med. 228, 197–206. https://doi.org/10.1016/j.freeradbiomed.2025.01.002 (2025).
Luo, X. et al. Plasma complement C3 and C3a are increased in major depressive disorder independent of childhood trauma. BMC Psychiatr. 22 (1), 741. https://doi.org/10.1186/s12888-022-04410-3 (2022).
Sanchez-Ruiz, J. A. et al. The Wnt signaling pathway in major depressive disorder: A systematic review of human studies. Psychiatr. Res. 339, 115983 (2024).
Sanchez-Ruiz, J. A. et al. The Wnt signaling pathway in major depressive disorder: A systematic review of human studies. Psychiatr. Res. 339, 115983. https://doi.org/10.1016/j.psychres.2024.115983 (2024).
Fan, H. et al. IRES-mediated Wnt2 translation in apoptotic neurons triggers astrocyte dedifferentiation. NPJ Regen Med. 7 (1), 42 (2022).
Zhang, D. et al. Wnt-3a alleviates neuroinflammation after ischemic stroke by modulating the responses of microglia/macrophages and astrocytes. Int. Immunopharmacol. 75, 105760. https://doi.org/10.1016/j.intimp.2019.105760 (2019).
Yang, C., Sui, G., Wang, L., Chen, Z. & Wang, F. MiR-124 prevents the microglial Proinflammatory response by inhibiting the activities of TLR4 and downstream NLRP3 in palmitic acid-treated BV2 cells. J. Mol. Neurosci. 72 (3), 496–506 (2022).
Tang, Y. et al. Berberine exerts antidepressant effects in vivo and in vitro through the PI3K/AKT/CREB/BDNF signaling pathway. Biomed. Pharmacother. 170, 116012 (2024).
Wang, W. et al. Effects of high-fat diet and chronic mild stress on depression-like behaviors and levels of inflammatory cytokines in the hippocampus and prefrontal cortex of rats. Neuroscience 480, 178–193 (2022).
Chiba, S. et al. Chronic restraint stress causes anxiety- and depression-like behaviors, downregulates glucocorticoid receptor expression, and attenuates glutamate release induced by brain-derived neurotrophic factor in the prefrontal cortex. Prog. Neuropsychopharmacol. Biol. Psychiatr. 39 (1), 112–119 (2012).
Mao, Y., Xu, Y. & Yuan, X. Validity of chronic restraint stress for modeling anhedonic-like behavior in rodents: a systematic review and meta-analysis. J. Int. Med. Res. 50 (2), 3000605221075816 (2022).
Zhuang, H. et al. Long-term high-fat diet consumption by mice throughout adulthood induces neurobehavioral alterations and hippocampal neuronal remodeling accompanied by augmented microglial lipid accumulation. Brain Behav. Immun. 100, 155–171 (2022).
Yao, D. et al. Melatonin alleviates depression-like behaviors and cognitive dysfunction in mice by regulating the circadian rhythm of AQP4 polarization. Transl Psychiatr. 13 (1), 310 (2023).
Bai, Y. et al. Engagement of N6-methyladenisine methylation of Gng4 mRNA in astrocyte dysfunction regulated by CircHECW2. Acta Pharm. Sin. B 14 (4), 1644–1660 (2024).
Lei, L., Wang, Y. T., Hu, D., Gai, C. & Zhang, Y. Astroglial connexin 43-mediated gap junctions and hemichannels: potential antidepressant mechanisms and the link to neuroinflammation. Cell. Mol. Neurobiol. 43 (8), 4023–4040 (2023).
Jiang, D. et al. Neuron-derived exosomes-transmitted miR-124-3p protect traumatically injured spinal cord by suppressing the activation of neurotoxic microglia and astrocytes. J. Nanobiotechnol. 18 (1), 105 (2020).
Anderson, M. A. et al. Astrocyte Scar formation aids central nervous system axon regeneration. Nature 532 (7598), 195–200 (2016).
Stym-Popper, G. et al. Regulatory T cells decrease C3-positive reactive astrocytes in Alzheimer-like pathology. J. Neuroinflamm. 20 (1), 64 (2023).
Cobb, J. A. et al. Density of GFAP-immunoreactive astrocytes is decreased in left hippocampi in major depressive disorder. Neuroscience 316, 209–220. https://doi.org/10.1016/j.neuroscience.2015.12.044 (2016).
O’Leary, L. A. & Mechawar, N. Implication of cerebral astrocytes in major depression: A review of fine neuroanatomical evidence in humans. Glia 69 (9), 2077–2099. https://doi.org/10.1002/glia.23994 (2021).
Zhang, J., Ning, L. & Wang, J. Dietary Quercetin attenuates depressive-like behaviors by inhibiting astrocyte reactivation in response to stress. Biochem. Biophys. Res. Commun. 533 (4), 1338–1346. https://doi.org/10.1016/j.bbrc.2020.10.016 (2020).
Zhang, H. Y. et al. A1 astrocytes contribute to murine depression-like behavior and cognitive dysfunction, which can be alleviated by IL-10 or fluorocitrate treatment. J. Neuroinflamm. 17 (1), 200 (2020).
Zhai, L. et al. Atractylenolide I suppresses A1 astrocyte activation to improve depression in mice. Mol. Neurobiol. 61 (9), 7037–7045 (2024).
Clyburn, C. & Browning, K. N. Role of astroglia in diet-induced central neuroplasticity. J. Neurophysiol. 121 (4), 1195–1206. https://doi.org/10.1152/jn.00823.2018 (2019).
Santiago-Balmaseda, A. et al. Neurodegenerative diseases: unraveling the heterogeneity of astrocytes. Cells 13 (11), 921. https://doi.org/10.3390/cells13110921 (2024).
Sonn, I., Nakamura, M., Renault-Mihara, F. & Okano, H. Polarization of reactive astrocytes in response to spinal cord injury is enhanced by M2 macrophage-mediated activation of Wnt/β-catenin pathway. Mol. Neurobiol. 57 (4), 1847–1862 (2020).
García-Velázquez, L., Alobayan, R., Morales-Moreno, D., Ávila-Muñoz, E. & Arias, C. Differential changes in Wnt7 and Dkk1 levels in astrocytes exposed to glutamate or TNFα. Neuroreport 35 (8), 542–550 (2024).
Sun, S. et al. WNT signaling represses astrogliogenesis via Ngn2-dependent direct suppression of astrocyte gene expression. Glia 67 (7), 1333–1343 (2019).
Tran, K. A. et al. Endothelial β-catenin signaling is required for maintaining adult blood-brain barrier integrity and central nervous system homeostasis. Circulation 133 (2), 177–186 (2016).
Li, H. et al. circFGFR4 promotes differentiation of myoblasts via binding miR-107 to relieve its inhibition of Wnt3a. Mol. Ther. Nucleic Acids 11, 272–283 (2018).
Meng, F. et al. The phenotype changes of astrocyte during different ischemia conditions. Brain Sci. 14 (12), 1256. https://doi.org/10.3390/brainsci14121256 (2024).
Zhou, W. J. et al. The antidepressant roles of Wnt2 and Wnt3 in stress-induced depression-like behaviors. Transl. Psychiatr. 6 (9), e892 (2016).
Dai, J. et al. Influence of miR-155 on behaviors of depression mice through regulating Wnt/β-catenin signaling pathway. Eur. Rev. Med. Pharmacol. Sci. 24 (3), 1398–1407 (2020).
Cook, I. A. et al. Time course of changes in peripheral blood gene expression during medication treatment for major depressive disorder. Front. Genet. 10, 870. https://doi.org/10.3389/fgene.2019.00870 (2019).
Lu, C. L. et al. Glucocorticoid receptor-dependent astrocytes mediate stress vulnerability. Biol. Psychiatr. 92 (3), 204–215. https://doi.org/10.1016/j.biopsych.2021.11.022 (2022).
Tsai, S. F. et al. High-fat diet suppresses the astrocytic process arborization and downregulates the glial glutamate transporters in the hippocampus of mice. Brain Res. 1700, 66–77. https://doi.org/10.1016/j.brainres.2018.07.017 (2018).
SofroniewMV Astrocyte barriers to neurotoxic inflammation [published correction appears. Nat. Rev. Neurosci. 16 (5), 249–263. https://doi.org/10.1038/nrn3898 (2015).
Liu, L., Yang, J., Qian, F. & Lu, C. Hypothalamic-pituitary-adrenal axis hypersensitivity in female rats on a post-weaning high-fat diet after chronic mild stress. Exp. Ther. Med. 14 (1), 439–446. https://doi.org/10.3892/etm.2017.4498 (2017).
Hassan, A. M. et al. High-fat diet induces depression-like behaviour in mice associated with changes in microbiome, neuropeptide Y, and brain metabolome. Nutr. Neurosci. 22 (12), 877–893 (2019).
Turnbaugh, P. J. et al. A core gut Microbiome in obese and lean twins. Nature 457 (7228), 480–484. https://doi.org/10.1038/nature07540 (2009).
Tang, C. F. et al. Short-chain fatty acids ameliorate depressive-like behaviors of high fructose-fed mice by rescuing hippocampal neurogenesis decline and blood-brain barrier damage. Nutrients 14 (9), 1882 (2022).
Li, Y. et al. TSHR signaling promotes hippocampal dependent memory formation through modulating Wnt5a/β-catenin mediated neurogenesis. Biochem. Biophys. Res. Commun. 704, 149723 (2024).
Jo, D., Yoon, G., Lim, Y., Kim, Y. & Song, J. Profiling and cellular analyses of obesity-related circrnas in neurons and glia under obesity-like in vitro conditions. Int. J. Mol. Sci. 24 (7), 6235 (2023).
Zhu, Z. H., Yang, R., Fu, X., Wang, Y. Q. & Wu, G. C. Astrocyte-conditioned medium protecting hippocampal neurons in primary cultures against corticosterone-induced damages via PI3-K/Akt signal pathway. Brain Res. 1114 (1), 1–10 (2006).
Wang, L. et al. Inulin attenuates blood-brain barrier permeability and alleviates behavioral disorders by modulating the TLR4/MyD88/NF-κB pathway in mice with chronic stress. J. Agric. Food Chem. 71 (36), 13325–13337 (2023).
Steru, L., Chermat, R., Thierry, B. & Simon, P. The tail suspension test: a new method for screening antidepressants in mice. Psychopharmacol. (Berl) 85 (3), 367–370 (1985).
Yoshizaki, K., Asai, M. & Hara, T. High-fat diet enhances working memory in the Y-maze test in male C57BL/6J mice with less anxiety in the elevated plus maze test. Nutrients 12 (7), 2036 (2020).
Shang, X., Shang, Y., Fu, J. & Zhang, T. Nicotine significantly improves chronic stress-induced impairments of cognition and synaptic plasticity in mice. Mol. Neurobiol. 54 (6), 4644–4658 (2017).
Funding
This work was supported by the STI2030-Major Projects-2021ZD0200700; Natural Science Foundation of Hebei Province (Grant Number H2024209070); Research Project of basic Scientific Research Service fees in Provincial Universities of North China University of Technology (Grant Number JQN2023039); Medical Science Research Project of Hebei Province in 2023 (Grant Number 20231585); Key project of Hebei Natural Science Foundation Traditional Chinese Medicine Joint Fund (Grant Number H2022209080); The central government guides local science and technology development fund project (Grant Number 246Z2511G).
Author information
Authors and Affiliations
Contributions
Ran Ding: Conceptualization, Methodology, Data curation, Visualization, Writing original draft, Writing—review & editing. Linyin Gao: Data curation, Methodology, Writing—review & editing. Xindi Wang: Methodology, Writing—review & editing. Jinxia Yang: Data curation. Xuemei Han: Data curation. Shuailong Fei: Data curation. Jian Wang: Data curation. Xiujun Zhang: Funding acquisition. Haitao Wang: Project administration, Funding acquisition Xueliang Shang: Methodology, Funding acquisition, Writing—review & editing. Lei Wu: Conceptualization, Methodology, Funding acquisition, Supervision, Project administration, Writing—review & editing.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Ding, R., Gao, L., Wang, X. et al. High-fat diet and chronic restraint stress exacerbate anxiety-depressive behaviors via astrocytic A1 phenotype transformation. Sci Rep 15, 15031 (2025). https://doi.org/10.1038/s41598-025-99355-4
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-99355-4