Abstract
The central amygdala (CeA) plays a crucial role in defensive and appetitive behaviours. It contains genetically defined GABAergic neuron subpopulations distributed over three anatomical subregions, capsular (CeC), lateral (CeL), and medial (CeM). The roles that these molecularly- and anatomically-defined CeA neurons play in appetitive behavior remain unclear. Using intersectional genetics in mice, we found that neurons driving food or water consumption are confined to the CeM. Separate CeM subpopulations exist for water only versus water or food consumption. In vivo calcium imaging revealed that CeMHtr2a neurons promoting feeding are responsive towards appetitive cues with little regard for their physical attributes. CeMSst neurons involved in drinking are sensitive to the physical properties of salient stimuli. Both CeM subtypes receive inhibitory input from CeL and send projections to the parabrachial nucleus to promote appetitive behavior. These results suggest that distinct CeM microcircuits evaluate liquid and solid appetitive stimuli to drive the appropriate behavioral responses.
Similar content being viewed by others
Introduction
The central amygdala (CeA), is a brain hub for emotion and motivation that rapidly integrates salient environmental and internal stimuli to generate appropriate behavioral responses1,2,3,4. While traditionally thought to be linked solely to defensive behavior, emerging evidence suggests that specific CeA neuronal populations play key roles in reward and consummatory behaviors, such as feeding5,6 and drinking7. Since food and water are intrinsically rewarding8,9, appetitive CeA neurons are positive valence neurons and the animals seek to promote the activation of these neurons. In vivo recordings have shown that appetitive CeA neurons respond to appetitive stimuli, such as food and water, as well as to cues predictive of these stimuli1,2,5,10,11,12. In vivo single-cell calcium imaging experiments revealed marked heterogeneity in specific appetitive subpopulations, with some appetitive neurons becoming selectively activated by appetitive cues, with others becoming inhibited or showing dynamic responses to multiple stimuli5,12,13. In addition to environmental stimuli, appetitive CeA neurons are activated by internal signals, such as the hunger hormone ghrelin13. Despite recent progress, the circuit mechanisms in the central amygdala that process appetitive signals such as food and water are still not well understood2,14,15,16,17.
The CeA consists exclusively of γ-aminobutyric acid-releasing (GABAergic) neurons that can be divided into subpopulations based on their molecular, morphological, physiological18, and functional properties. They are organized in inhibitory microcircuits in three subregions of the CeA: the central capsular (CeC), lateral (CeL), and medial subregions (CeM). For defensive behavior, the information flow through the subregions has been partially characterized: CeC and CeL subregions are the primary targets for sensory inputs that are processed and passed on to the CeM from where major projections go to hindbrain autonomic and motor control areas2,19,20. For appetitive behavior, the information flow is less clear. New perspectives have been suggested, with the CeL also forming long-range efferent projections bypassing the CeM, and the CeM receiving direct inputs bypassing the CeL21,22.
The functional characterization of molecularly-defined appetitive CeA subpopulations has been the subject of recent intense research. It has mostly relied on the manipulation of single Cre driver lines and expression analysis of selected markers5,7,23,24,25. The more recent characterization of CeA subpopulations by scRNAseq analysis revealed that several of these Cre drivers are expressed in more than one cell cluster and, importantly, across CeA subdivisions13,22,26. The Sst-Cre driver has been extensively used to demonstrate that CeA neurons expressing the neuropeptide somatostatin, control both appetitive and aversive behaviors7,11,12,27,28. In vivo calcium imaging suggested that Sst+ CeA neurons help discriminate between stimuli with different physical properties and participate in the evaluation of salient events during reward learning12. CeASst neurons can be found in CeL and CeM subregions and partially overlap with neurons expressing neurotensin (Nts)7,29 which show a similar distribution pattern and promote consumption of palatable fluids24. The anatomical position of appetitive CeASst and CeANts neurons has not been fully unraveled.
In previous work, we have shown that CeA neurons expressing the Htr2a-Cre driver promote food consumption through a positive valence signal5. CeAHtr2a neurons are activated by fasting, the hunger hormone ghrelin, and the presence of food13. Single-cell transcriptomics revealed that CeAHtr2a neurons are located in the CeL and CeM and it is currently unclear which of these populations promotes food consumption. A large fraction of Htr2a-Cre-expressing CeA neurons overlaps with neurons expressing prepronociceptin (Pnoc)13. Similar to CeAHtr2a neurons, CeAPnoc neurons promote palatable food consumption23, but the exact anatomical location of Pnoc-positive appetitive neurons remains unclear. CeAHtr2a neurons located in the CeL overlap with the Sst-positive population, whereas CeMHtr2a and CeMSst neurons are largely separate populations13.
To better define the CeA appetitive microcircuits, we employed combinatorial recombinase-dependent targeting30 using available Cre lines in combination with a transgenic line that expresses the Flp recombinase specifically in neurons of the CeL subregion. This approach, in combination with INTRSECT Boolean vectors31,32, allowed for functional characterization of four spatially and molecularly distinct CeA subpopulations, CeLSst, CeMSst, CeLHtr2a, and CeMHtr2a neurons. The results revealed the organization of appetitive information flow through the CeA. Our results indicate that neurons driving food or water consumption are confined to the CeM. Separate CeM subpopulations exist for water only (CeMSst), and water or food consumption (CeMHtr2a). In vivo calcium imaging revealed that CeMHtr2a neurons promoting feeding or drinking are highly responsive towards appetitive cues with little regard for their physical attributes. CeMSst neurons involved exclusively in drinking are sensitive to the physical properties of salient stimuli. Both CeM subtypes are controlled by inhibitory signals from the CeL and, in turn, form long-range inhibitory projections to the PBN to promote water or food consumption. These results suggest that distinct CeM microcircuits evaluate liquid and solid appetitive stimuli to drive the appropriate behavioral responses.
Results
A FlpoER transgenic line for intersectionally targeting central amygdala neurons
To manipulate CeL versus CeM neurons with an intersectional genetics strategy, we generated a Wfs1-FlpoER mouse line that expresses the tamoxifen-inducible optimized FlpoER recombinase under the control of the Wolframin1 (Wfs1) promoter (Fig. 1a). Wfs1 was previously shown to mark the majority of cells in the CeL33,34 and we confirmed that Wfs1 immunoreactivity was enriched in the CeL (Fig. 1b). Using Htr2a-Cre and Sst-Cre driven tdTomato reporter mice, we found that nearly 90% of Htr2a-Cre and Sst-Cre-positive cells expressed Wfs1 in the CeL, and a large fraction of Wfs1 immunoreactive cells encompassed Htr2a-Cre and Sst-Cre-positive cells (Supplementary Fig. 1a–j). To validate the accuracy of FlpoER expression, we bred Wfs1-FlpoER mice with an FPDI reporter line which expresses mCherry after Flp-mediated recombination35. After three tamoxifen injections, 56% of cells in CeL that were immunopositive for endogenous Wfs1 expressed FlpoER (mCherry), and, importantly for our intersectional targeting strategy, mCherry+ cells were 17-times more abundant in CeL than CeM (Supplementary Fig. 1k–o).
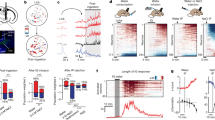
a Wfs1-FlpoER BAC transgene. Abbreviations: FlpoER optimized Flp-estrogen receptor fusion protein, NLS nuclear localization sequence, WPRE-pA woodchuck hepatitis post-transcriptional regulatory element with bovine growth hormone polyadenylation signal, gb2neo gb2 prokaryotic promoter driven Neo cassette. b Example showing endogenous Wfs1 immunoreactivity in the CeL. c Intersectional strategy used with Htr2a-Cre;Wfs1-FlpoER and Sst-Cre;Wfs1-FlpoER mice. Cells in CeL are either FlpoER positive or FlpoER and Cre double positive. Cells in CeM are only Cre-positive. d Examples of INTRSECT viruses. Con/Fon viruses express in CeL cells that are double positive for Cre and FlpoER, while Con/Foff viruses express in CeM cells that are Cre-positive and FlpoER-negative. e, t Expression through anterior-posterior axis [Bregma −0.9 (e, i, m, q) and −1.2 (f, j, n, r)] of Cre-dependent AAV-DIO-mCherry (not intersectional, red) in Htr2a-Cre;Wfs1-FlpoER mice showing the entire population of CeAHtr2a cells in the CeL and CeM (e, f), and expression of AAV-Con/Fon-EYFP (green) in the same mice predominantly highlighting the CeL fraction of Htr2a positive cells (e, f). Quantifications of the fraction of Htr2a-Cre::Con/Fon-EYFP positive cells in the CeL (54.3 ± 7.5%) among all CeAHtr2a cells in CeL (g) and distribution of Htr2a-Cre;Con/Fon-EYFP positive cells in CeL and CeM (h). i, j Expression of AAV-Con/Foff-EYFP (CeM enriched, green) versus AAV-DIO-mCherry (red) as described for (e, f). Quantifications of the fractions of Htr2a-Cre::Con/Foff-EYFP positive cells in CeM (66.3 ± 2.0%) among all CeAHtr2a cells in CeM (k) and distribution of Htr2a-Cre;Con/Foff-EYFP positive cells in CeL and CeM (l). m, n Expression of AAV-Con/Fon-EYFP (CeL enriched, green) versus AAV-DIO-mCherry (red) in Sst-Cre;Wfs1-FlpoER mice. o, p Quantification of the fraction of Sst-Cre::Con/Fon-EYFP positive cells in CeL (47.5 ± 7.7%) among all Sst-positive cells in CeL (o) and distribution of Sst-Cre;Con/Fon-EYFP positive cells in CeL and CeM (p). q, r Expression of AAV-Con/Foff-EYFP (CeM enriched, green) versus AAV-DIO-mCherry (red) in Sst-Cre;Wfs1-FlpoER mice. Quantification of the fraction of Sst-Cre::Con/Foff-EYFP positive cells in CeM (68.6 ± 5%) among all CeASst cells in CeM (s) and distribution of Sst-Cre;Con/Foff-EYFP positive cells in CeL and CeM (t) (all groups: n = 3 mice/ 9 sections). Values = Mean ± SEM. Scale bar: 115 μm.
Crossing Htr2a-Cre or Sst-Cre with Wfs1-FlpoER mice generated double transgenic “intersectional mice” (Htr2a-Cre;Wfs1-FlpoER and Sst-Cre;Wfs1-FlpoER), in which most cells in the CeL should express both Cre and FlpoER, while the vast majority of cells in the CeM should only express Cre and not FlpoER (Fig. 1c). Injection of INTRSECT Boolean vectors31,32 into the CeA of these mice made it possible to specifically manipulate either the CeL (CreON/FlpON virus or Con/Fon in short) or the CeM population (CreON/FlpOFF virus or Con/Foff) (Fig. 1d). To validate the fidelity of this approach, we injected either Con/Fon or Con/Foff EYFP virus into the CeA of Htr2a-Cre;Wfs1-FlpoER mice (together with a Cre-dependent mCherry virus to visualize the entire Cre-positive population). The results with the Con/Fon-EYFP virus showed that EYFP+ cells were more abundant in the CeL than CeM across the anterior-posterior extent of the CeA (Fig. 1e, f). Quantifications revealed that among the Htr2a-Cre-positive cells in the CeL, the majority (54%) expressed EYFP (Fig. 1g) and 4-times as many EYFP cells (80%) were present in CeL versus CeM (Fig. 1h). Conversely, with the Con/Foff virus, EYFP+ cells were more abundant in the CeM than the CeL (Fig. 1i, j). Quantifications revealed that among the Htr2a-Cre-positive cells in the CeM, the majority (66%) expressed EYFP (Fig. 1k), and 2.3-times as many EYFP cells (70%) were present in CeM versus CeL (Fig. 1l). Similar results were obtained with Sst-Cre;Wfs1-FlpoER mice. With the Con/Fon-EYFP virus, EYFP+ cells were more abundant in the CeL than CeM (Fig. 1m, n). Among Sst-Cre-positive cells in the CeL a large fraction (48%) expressed EYFP (Fig. 1o) and 10 times as many EYFP cells (91%) were present in CeL versus CeM (Fig. 1p). With the Con/Foff virus, EYFP+ cells were more abundant in the CeM than CeL (Fig. 1q, r). Among the Sst-Cre-positive cells in the CeM the majority (69%) expressed EYFP (Fig. 1s) and 2.7 times as many EYFP cells (71%) were present in CeM versus CeL (Fig. 1t). These experiments show that our Cre/Flp intersectional mouse model, combined with INTRSECT viruses, enabled an enrichment of actuator expression in a specific subpopulation within a single CeA subregion.
Promotion of water intake exclusively by CeM subpopulations
To study the role of different CeA subpopulations in water consumption, we first asked if optogenetic activation of the neurons would be sufficient to promote water intake. We stereotactically injected the Con/Fon- or Con/Foff-hChR2-EYFP viruses bilaterally into the CeA of Htr2a-Cre;Wfs1-FlpoER mice to express channelrhodopsin (ChR2) in the CeL and CeM subpopulations, respectively (Fig. 2a). Similar INTRSECT viruses expressing only EYFP control protein were used as negative controls. As positive controls, we expressed ChR2 in the entire CeAHtr2a population by including one cohort of Htr2a-Cre mice that was injected with the simple Cre-dependent DIO-hChR2-EYFP virus (Fig. 2a). Similar injections were performed with Sst-Cre;Wfs1-FlpoER and Sst-Cre mice to analyze the CeASst subpopulations. The expression of EYFP in different CeA subregions was validated in all animals (Fig. 2b–e). Optic fibers were placed bilaterally over the CeA to photoactivate the cell bodies of the neurons (Supplementary Fig. 2a–g). In a 30-min paradigm, thirsty animals were exposed to water while being bilaterally photoactivated throughout the session. The same experiment was repeated when animals were hydrated. We found that photoactivation of the entire CeAHtr2a and CeASst populations was sufficient to promote water consumption by thirsty as well as hydrated mice (Fig. 2f; Supplementary Fig. 3a). Surprisingly, among the two subpopulations, it was exclusively the CeM fractions of both cell types that promoted water intake both in thirsty and hydrated mice (Fig. 2g, h; Supplementary Fig. 3b, c).
a Viruses injected and optic fiber placement in the CeA (modified from Allen Mouse Brain Atlas, mouse.brain-map.org). Representative images of ChR2-EYFP expression in CeA subregions after injections of INTRSECT Con/Fon or Con/Foff viruses into Htr2a-Cre;Wfs1-FlpoER (b) or Sst-Cre;Wfs1-FlpoER mice (c). All mice used for behavior showed similar results. d, e Similar manipulations as in (b, c) except for INTRSECT expression of EYFP protein. f Water intake during photoactivation of all CeAHtr2a or CeASst neurons for 30-min by water-deprived mice compared to photoactivated controls (Htr2a: unpaired two-tailed t test p < 0.0001, t = 6.429. Sst: Mann-Whitney two-tailed U test p = 0.0124, U = 16). (CeAHtr2a: n = 9 Ctrl, n = 11 ChR2. CeASst: n = 8 Ctrl, n = 12 ChR2 mice). g Water intake during photoactivation of the CeL (Htr2a: unpaired two-tailed t test p = 0.0913, t = 1.774. Sst: unpaired two-tailed t test p = 0.7017 t = 0.3886). (CeLHtr2a: n = 10 Ctrl, n = 12 ChR2. CeLSst: n = 12 Ctrl, n = 10 ChR2 mice). h Water intake during photoactivation of the CeM (Htr2a: Mann-Whitney two-tailed U test p < 0.0001, U = 2. Sst: unpaired two-tailed t test p < 0.0001 t = 5.054). (CeMHtr2a: n = 11 Ctrl, n = 9 ChR2. CeMSst: n = 10 Ctrl, n = 11 ChR2 mice). i–l Photoactivation of CeMHtr2a (i) or CeMSst neurons (j) during alternating 10-min light OFF and ON epochs in water-deprived mice increased water intake during light ON epochs (Htr2a: 10–20 min: Mann-Whitney two-tailed U test p = 0.0002, U = 0. 30–40 min: unpaired two-tailed t test p = 0.0003, t = 4.801. 50–60 min: Mann-Whitney two-tailed U test p = 0.0030, U = 5.500. Sst: 10–20 min: unpaired two-tailed t test p = 0.0002, t = 4.738. 30–40 min: Mann-Whitney two-tailed U test p < 0.0001, U = 1.500. 50–60 min: Mann-Whitney two-tailed U test p = 0.0012, U = 8). Photoactivation of the CeL fractions (k, l) had no effect (Htr2a: 10–20 min: unpaired two-tailed t test p = 0.6180, t = 0.5069. 30–40 min: Mann-Whitney two-tailed U test p = 0.6326, U = 48. 50–60 min: Mann-Whitney two-tailed U test p = 0.4624, U = 49. Sst: 10–20 min: unpaired two-tailed t test p = 0.1053, t = 1.697. 30–40 min: Mann-Whitney two-tailed U test p = 0.4264, U = 48. 50–60 min: Mann-Whitney two-tailed U test p = 0.0697, U = 36). (CeMHtr2a: n = 7 Ctrl, n = 9 ChR2. CeMSst: n = 9 Ctrl, n = 10 ChR2. CeLHtr2a: n = 10 Ctrl, n = 11 ChR2. CeLSst: n = 12 Ctrl, n = 10 ChR2 mice). Values = Mean ± SEM. Scale bar: 115 μm.
To ensure consistency in the effects of optogenetic stimulation across multiple trials, we used a different drinking test during which short (10-min) periods of photoactivation alternated with periods of no photoactivation. During an initial 10-min Light-OFF period, water-deprived mice from all experimental groups were found to consume similar amounts of water (Fig. 2i–l). As the experiment progressed, control animals gradually became hydrated and consumed less water, independent of whether the light was ON or OFF. Among the experimental groups, only photoactivation of the entire CeA populations or the CeM subpopulations increased water consumption during each Light-ON period (Fig. 2i, j; Supplementary Fig. 3d, e). Photoactivation of the CeL subpopulations was insufficient to promote water consumption (Fig. 2k, l). Similar results were obtained with hydrated animals (Supplementary Fig. 3f–k). Overall consumption of water was highest during Light-ON periods for mice expressing ChR2 in the entire CeA or CeM subpopulations of Htr2a+ and Sst+ cells, while water consumption was unaffected in photoactivated controls and mice expressing ChR2 in the CeL (Supplementary Fig. 4). In summary, these experiments showed that the CeM subpopulations of Htr2a-Cre and Sst-Cre-positive cells were sufficient to drive water consumption.
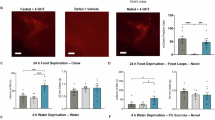
We next asked if the CeM subpopulations were also required for water consumption. We stereotactically injected AAVs expressing Cre-dependent eNpHR3.0 bilaterally into the CeA of Htr2a-Cre mice to express the inhibitory Halorhodopsin in the entire CeAHtr2a population (Fig. 3a), or similarly INTRSECT Con/Foff IC++, a blue-shifted chloride-conducting channelrhodopsin32,36, into Htr2a-Cre;Wfs1-FlpoER mice to express the actuator specifically in the CeM subpopulation (Fig. 3b). Similar injections were performed with Sst-Cre;Wfs1-FlpoER mice to analyze the CeMSst subpopulation (Fig. 3a, b). Corresponding Cre-dependent and INTRSECT viruses expressing mCherry or EYFP control proteins were injected as controls (Fig. 3a, b). Animals were water deprived and tested for water consumption for 30 min while being photoinhibited. Inhibition of the entire CeA population, as well as the respective CeMHtr2a and CeMSst subpopulations significantly decreased water intake compared to light-stimulated controls (Fig. 3c, d). Together, these experiments reveal that the CeM subpopulations of Htr2a-Cre and Sst-Cre-positive neurons are necessary for efficient water consumption in thirsty mice.
a Representative images showing expression of Cre-dependent eNpHR3.0 and control mCherry in the entire CeA of Htr2a-Cre and Sst-Cre animals. All mice used for behavior showed similar results. b Representative images showing expression of IC++ and control YFP predominantly in the CeM after injection of Con/Foff virus in Htr2a-Cre;Wfs1-FlpoER and Sst-Cre;Wfs1-FlpoER mice. All mice used for behavior showed similar results. c Photoinhibition of the entire populations of CeAHtr2a (blue) or CeASst neurons (green) in water-deprived mice significantly decreased water consumption compared to photoinhibited controls during a 30-min drinking assay (Htr2a: unpaired two-tailed t test p = 0.0007, t = 3.939. Sst: unpaired two-tailed t test p = 0.0363, t = 2.213). (CeAHtr2a: n = 12 Ctrl, n = 12 eNpHR3.0. CeASst: n = 13 Ctrl, n = 14 eNpHR3.0 mice). d Photoinhibition of the CeM fractions of CeAHtr2a (blue) or CeASst neurons (green) significantly decreased water consumption compared to photoinhibited controls in the same assay (Htr2a: unpaired two-tailed t test p = 0.0022, t = 3.578. Sst: unpaired two-tailed t test p = 0.0017, t = 3.733). (CeMHtr2a: n = 12 Ctrl, n = 12 IC++. CeMSst: n = 11 Ctrl, n = 9 IC++ mice). Values = Mean ± SEM. Scale bar: 115 μm.
Promotion of food intake exclusively by CeMHtr2a, but not CeMSst, neurons
Next, we focused our attention on feeding behavior. We previously reported that CeAHtr2a neurons stimulated food intake5,13, but the specific contributions of the CeL and CeM subpopulations had not been explored. Likewise, the roles of the different Sst subtypes in regulating feeding are not completely understood7. We performed a free-feeding assay with ad libitum fed animals expressing ChR2 in Htr2a-Cre+ or Sst-Cre+ neurons in the CeA, CeL, or CeM. As expected, photoactivation of the entire CeAHtr2a neuron population caused a significant increase in food intake compared to the photoactivated control group (Fig. 4a). Surprisingly, food intake was exclusively driven by the CeMHtr2a, but not the CeLHtr2a, subpopulation (Fig. 4b, c). Photoactivation of the entire CeASst neuron population or its subpopulations in the CeL and CeM failed to significantly increase food consumption under these conditions (Fig. 4a–c).
a Photostimulation of the entire population of CeAHtr2a neurons promoted feeding of satiated mice (two-tailed Wilcoxon signed-rank test p = 0.0078). No statistically significant effect after photostimulation CeASst neurons compared to controls (Mann-Whitney two-tailed U test p = 0.1821, U = 22). (CeAHtr2a: n = 8 Ctrl, n = 8 ChR2. CeASst: n = 7 Ctrl, n = 10 ChR2 mice). b Photostimulation of CeLHtr2a or CeLSst subpopulations did not promote feeding behavior (CeLHtr2a: Mann-Whitney two-tailed U test p = 0.1073, U = 30.50. CeLSst: p = 0.9183, U = 48). (CeLHtr2a: n = 10 Ctrl, n = 10 ChR2. CeLSst: n = 11 Ctrl, n = 9 ChR2 mice). c Photostimulation of CeMHtr2a, but not CeMSst neurons, increased food intake (CeMHtr2a: Mann-Whitney two-tailed U test p = 0.0235, U = 19.50. CeMSst: p = 0.2783, U = 41.50). (CeMHtr2a: n = 10 Ctrl, n = 9 ChR2. CeMSst: n = 10 Ctrl, n = 11 ChR2 mice). d Photoinhibition of the entire population of CeAHtr2a neurons in fed mice decreased the ingestion of a palatable liquid reward compared to controls (unpaired two-tailed t test p = 0.0170, t = 2.593). No effect by photoinhibition of the entire population of CeASst neurons (Mann-Whitney two-tailed U test p = 0.1285, U = 37). (CeAHtr2a: n = 12 Ctrl, n = 11 eNpHR3.0. CeASst: n = 14 Ctrl, n = 14 eNpHR3.0 mice). e Photoinhibition of CeMHtr2a, but not CeMSst, neurons decreased the consumption of a palatable liquid reward compared to controls (CeMHtr2a unpaired two-tailed t test p = 0.0005, t = 4.313; CeMSst unpaired two-tailed t test p = 0.5433, t = 0.6230). (CeMHtr2a: n = 9 Ctrl, n = 10 IC + +. CeMSst: n = 10 Ctrl, n = 6 IC + + mice). Values = Mean ± SEM.
To investigate if the activity of the CeMHtr2a subpopulation was required for efficient food consumption, we asked if photoinhibition of CeMHtr2a neurons would reduce consumption of a palatable liquid reward (Fresubin, 2 kcal/ml) as previously shown for the entire CeAHtr2a population5. Indeed, photoinhibition of the entire CeAHtr2a population or the CeMHtr2a subpopulation significantly reduced the consumption of the palatable liquid compared to the control groups (Fig. 4d, e). The effect size of photoinhibiting the CeMHtr2a subpopulation was larger compared to the entire CeAHtr2a population, with a Cohen’s d of 0.8, indicating a medium effect. In contrast, inhibition of the entire CeASst population or the CeMSst subpopulation did not have a significant effect on Fresubin consumption (Fig. 4d, e). These results provide genetic evidence for CeMHtr2a neurons promoting water and food consumption, whereas CeMSst neurons had a more restricted role in water, but not food consumption. The CeL subpopulations of Htr2a-Cre+ and Sst-Cre+ neurons, which highly overlap, do not seem to affect water or food consumption, at least in the assays tested.
CeM subpopulations drive real-time place preference and conditioned reward behavior
Next, we asked which subpopulations could drive innate rewarding, but non-consummatory behavior, in a real-time place preference (RTPP) assay consisting of a two-chamber arena with one compartment paired with laser photostimulation (Fig. 5a). The results revealed a similar pattern as for water intake: activation of the entire populations of CeAHtr2a or CeASst neurons as well as the respective CeM subpopulations resulted in the animals exhibiting a significant preference for the photoactivation-paired chamber, as compared to the CeL subpopulations and the controls (Fig. 5b–d). The time spent in the center of an open-field arena as well as the distance moved was unchanged, suggesting that the reward behavior in the RTPP assay was not due to an anxiolytic effect (Supplementary Fig. 5a–e).
a Real-time place preference paradigm. Representative heat map (generated by Ethovision) of a photostimulated CeMHtr2a::ChR2 mouse in the RTPP task. b Preference for the light-paired chamber when the entire populations of CeAHtr2a or CeASst neurons are activated in the RTPP task, compared to controls (CeAHtr2a: unpaired two-tailed t test p = 0.0010, t = 4.018. CeASst: unpaired two-tailed t test p = 0.0347, t = 2.409). (CeAHtr2a: n = 8 Ctrl, n = 10 ChR2. CeASst: n = 6 Ctrl, n = 7 ChR2 mice). c No preference for the light-paired chamber when only CeLHtr2a or CeLSst neurons are activated (Htr2a: unpaired two-tailed t test p = 0.1521, t = 1.492, Sst: unpaired two-tailed t test p = 0.3857, t = 0.8879). (CeLHtr2a: n = 9 Ctrl, n = 12 ChR2. CeLSst: n = 12 Ctrl, n = 9 ChR2 mice). d Preference for the light-paired chamber when only CeMHtr2a or CeMSst neurons are activated (Htr2a: unpaired two-tailed t test p = 0.0013, t = 3.844, Sst: unpaired two-tailed t test p = 0.0383, t = 2.271). (CeMHtr2a: n = 10 Ctrl, n = 9 ChR2. CeMSst: n = 8 Ctrl, n = 9 ChR2 mice). e Conditioned flavor preference test. f Conditioned flavor preference index of mice in which the entire populations of CeAHtr2a (blue) or CeASst (green) neurons were photostimulated in comparison to control mice (CeAHtr2a: main effect ChR2, Two-way ANOVA, F(1,16) = 10.12, p = 0.0058; Bonferroni post-hoc test p < 0.0001. CeASst: main effect ChR2, Two-way ANOVA, F(1,18) = 32.06, p < 0.0001; Bonferroni post-hoc test p = 0.0057). (CeAHtr2a: n = 7 Ctrl, n = 11 ChR2. CeASst: n = 10 Ctrl, n = 10 ChR2 mice). g Conditioned flavor preference index of mice in which only the CeLHtr2a (blue) or CeLSst (green) neurons were photostimulated (CeLHtr2a: main effect ChR2, Two-way ANOVA, F(1,19) = 5.611, p = 0.0286; Bonferroni post-hoc test p = 0.0313. CeLSst: main effect Time, Two-way ANOVA, F(1,20) = 4.918, p = 0.0383; Bonferroni post-hoc test p = 0.0255). (CeLHtr2a: n = 9 Ctrl, n = 12 ChR2. CeLSst: n = 12 Ctrl, n = 10 ChR2 mice). h Conditioned flavor preference index of mice in which only the CeMHtr2a (blue) or CeMSst (green) neurons were photostimulated (CeMHtr2a: main effect ChR2, Two-way ANOVA, F(1,19) = 10.05, p = 0.0050; Bonferroni post-hoc test p < 0.0001. CeMSst: main effect ChR2: Two-way ANOVA, F(1,17) = 8.165, p = 0.0109; Bonferroni post-hoc test p < 0.0001). (CeMHtr2a: n = 10 Ctrl, n = 11 ChR2. CeMSst: n = 8 Ctrl, n = 11 ChR2 mice). Values = Mean ± SEM.
Further, we investigated which subpopulation might condition a preference for a specific flavor. In a reward conditioning paradigm, pairing activation of appetitive CeA neurons with one of two flavors can reverse flavor preference, such that an initially less preferred flavor becomes the preferred one5,17. Mice expressing ChR2 in the entire populations of CeAHtr2a or CeASst neurons, or the respective CeM subpopulations, were allowed to consume two differently flavored nonnutritive liquids. After determining their individual baseline preference, conditioning was performed by pairing the less preferred flavor with optogenetic activation (Fig. 5e). After conditioning, the flavor preference of the mice was assessed by simultaneously offering liquids of both flavors. The results showed that activation of the CeM subpopulations of CeAHtr2a or CeASst neurons, reversed the animals’ initial preference, resulting in the least preferred flavor becoming the preferred one (Fig. 5f–h). Somewhat surprisingly, also the CeL subpopulations showed significant conditioning activities (Fig. 5g). Consistent with these results, mice expressing ChR2 in the CeM subpopulations of CeAHtr2a or CeASst neurons consumed a significantly larger amount of the liquid that was paired with photoactivation compared to controls (Supplementary Fig. 5f–h). These results demonstrate that the activities of both CeMHtr2a and CeMSst subpopulations are intrinsically positively reinforcing in RTPP and conditioned flavor preference assays. Both, CeLHtr2a and CeLSst subpopulations displayed modest reinforcing activity in the conditioned flavor preference assay, but perhaps not in real-time place preference.
CeMHtr2a and CeMSst neuron responses to different rewarding behaviors
To understand how CeMHtr2a and CeMSst neurons participate in consummatory behavior, we performed single-cell resolution in vivo calcium imaging in freely behaving mice. We injected a Con/Foff-GCaMP6m virus unilaterally into the CeA of either Htr2a-Cre::Wfs1-FlpoER or Sst-Cre::Wfs1-FlpoER animals to record the neuronal activity preferentially from CeM subpopulations. A gradient-index (GRIN) lens was implanted to monitor the neuronal activity using a head-mounted miniscope. We tested the animals in three separate conditions: exposure to water, food, and Fresubin. To make consumption of water and food pleasant, mice were water- and food-deprived, respectively. Fresubin instead is very palatable independent of the hunger state. Hence, mice exposed to Fresubin were fed ad libitum. Recordings were done for 10 min following a 10 min habituation period (Fig. 6a, b). During habituation, we recorded the activities of an average of 128 neurons per subpopulation, while during stimulus exposure, the numbers of active neurons increased to an average of 160 neurons (Supplementary Fig. 6a). The average number of neurons that were active across different rewarding conditions ranged from 78 to 114 (Supplementary Fig. 6b). When comparing the activities of all active cells during habituation and stimulation (including active and inactive times), we observed a general increase in neuronal activity for both CeMHtr2a and CeMSst neurons during stimulus exposure, except for CeMHtr2a neurons exposed to water (Fig. 6c, d). When comparing the activities during reward consumption versus inactive episodes during the 10-min stimulation periods, we found that CeMHtr2a and CeMSst neurons showed higher activity during drinking and feeding (Supplementary Fig. 6c).
a Behavioral paradigm. b Maximum projection images of calcium responses from CeMHtr2a neurons during habituation and drinking. c Representative traces from CeMHtr2a neurons during reward consumption. Consumption bouts in gray. d Average z-score comparisons of the general activity of CeMHtr2a and CeMSst neurons during habituation and reward (Mann-Whitney two-tailed U test. CeMHtr2a: water, p = 0.1771, U = 7322, n = 112–145; food, p = 0.0098, U = 9440, n = 135–169; Fresubin, p = 0.0001, U = 4901, n = 111–124. CeMSst: water, p < 0.0001, U = 8130, n = 143–176; food, p < 0.0001, U = 7320, n = 128–176; Fresubin p < 0.0001, U = 8119, n = 138–168). e 3D Visualization of CEBRA-generated embeddings of CeMHtr2a neurons considering consumption behavior and neural dynamics over time. Shuffled: drinking data. The three dimensions represent the three CEBRA principle components. Each dot represents a time point. Yellow and purple dots mark consumption and resting times, respectively. Comparison of behavioral decoding accuracy using a Random Forest (RF) algorithm across animals for CeMHtr2a (f) and CeMSst neurons (g) (drinking, feeding, Fresubin: two-tailed Wilcoxon matched-pairs signed-rank test p < 0.0001). (Prediction scores of accuracy of behavior compared to shuffled data: Actual-Shuffled Htr2a, n = 25; Sst, n = 36). h CEBRA-generated embeddings of CeMHtr2a neurons that were longitudinally detected during drinking and feeding, and during drinking and Fresubin consumption. Accuracy of behavioral decoding through RF, employing CEBRA embeddings of CeMHtr2a (i) and CeMSst (j) neurons longitudinally detected during two behaviors (paired two-tailed t test. CeMHtr2a: drinking-feeding: p = 0.0158, t = 4.956; feeding-drinking: p = 0.0175, t = 4.768; drinking-Fresubin: p = 0.0292, t = 3.935; Fresubin-drinking: p = 0.0337, t = 3.724. CeMSst: drinking-feeding: p = 0.0003, t = 6.068; feeding-drinking: p = 0.0007, t = 5.372; drinking-Fresubin: p = 0.0011, t = 4.955; Fresubin-drinking: p = 0.0012, t = 4.867). (Prediction scores of accuracy of behavior compared to shuffled data: Actual-Shuffled Htr2a, n = 4; Sst, n = 9). k Chord diagrams and bar graphs depicting stable or unstable (gray) correlations of the same CeMHtr2a and CeMSst neurons detected during drinking and feeding (Htr2a: stable v.s. unstable: unpaired two-tailed t test p = 0.5418, t = 0.6371, n = 5 mice. Sst: p = 0.0193, t = 2.602, n = 9 mice). l Similar analysis for CeMHtr2a neurons detected during feeding and Fresubin sessions (stable v.s. unstable: unpaired t test p = 0.0017, t = 4.627, n = 5 mice). Values = Mean ± SEM.
To investigate whether consummatory behaviors contributed to the dynamics of neural activity, we analyzed the data using the recently developed machine learning algorithm CEBRA37 (for further details, see “Methods”). We applied the so-called Hybrid model, which considers the association between consumption bouts and the patterns of neural activity across time. If a behavior contributed significantly to neural activity, we expected to see clear structure in CEBRA-generated 3D neural embeddings compared to shuffled embeddings. When applying CEBRA to both CeMHtr2a and CeMSst calcium recordings during drinking, feeding, and Fresubin consumption, we found clear structures for all three behaviors, i.e., a separation of data points derived from times when the animals were engaged in consummatory behavior from times when the animals were not involved in consumption (Fig. 6e). Notably, this separation was lost when the data was shuffled, suggesting contributions of these behaviors to neural activity (Fig. 6e). To analyze how well the neural embeddings decoded specific behavioral features, we applied a Random Forest (RF) classifier for behavioral decoding (see “Methods”). The results indicated that the CeMHtr2a and CeMSst neural embeddings decoded the respective behaviors with 85–90% accuracy, significantly better than shuffled data (60–75%) (Fig. 6f, g). To measure the prediction error of the RF classifier, we calculated the out of bag (OOB) error, and found that in all cases the OOB error from the actual data was much lower than from shuffled data (Supplementary Fig. 6d). Next, we asked if neurons associated with different behaviors were preserved across contexts with shared activity, using longitudinal registration of recorded neurons. We applied CEBRA to neural activity data of neurons shared between two behaviors (e.g., drinking/feeding, drinking/Fresubin). We found that the neurons associated with both behaviors showed clearly structured CEBRA embeddings (Fig. 6h, Supplementary Fig. 6e). To analyze how well the neural embeddings decoded specific behavioral features, we applied the RF classifier. The embeddings derived from drinking behavior were used for predicting feeding and those from feeding for predicting drinking. Furthermore, the drinking embeddings were applied to predict Fresubin consumption and vice versa. The results revealed that the neural embeddings decoded the behavioral predictions with higher accuracy compared to the results obtained with shuffled data (Fig. 6i, j, Supplementary Fig. 6f).
Through correlation analysis between behavior and neuronal activity, we found ensembles of CeMHtr2a and CeMSst populations whose activities correlated positively and negatively with reward consumption (Supplementary Fig. 6g). The CeMSst populations responded rather homogeneously to stimulus exposure, with 46–48% of cells displaying positive correlation with all three rewards (Supplementary Fig. 6h). The responses of CeMHtr2a neurons were more heterogenous, with 39–59% showing positive correlation (Supplementary Fig. 6h). The highest positive correlation of CeMHtr2a neurons was with feeding (59%), consistent with their observed function in food consumption.
Next, we asked how the activity of individual cells changed between the rewards having different physical properties. We compared all cells that were active during two behavioral sessions, e.g., drinking and feeding or feeding and Fresubin, and asked which fractions of cells kept their correlated activity constant (positive–positive, or negative–negative correlation) versus fractions that changed their activity (Fig. 6k,l; Supplementary Fig. 6j). When comparing drinking and consuming solid food, the fraction of CeMHtr2a cells displaying a stable correlation (pos–pos, neg–neg) was comparable to the fraction of cells that changed their activity (47% versus 53%) (Fig. 6k). Instead, the fraction of CeMSst cells that changed their activity was significantly larger than the fraction of cells displaying a stable correlation (63% versus 37%) (Fig. 6k). When comparing solid food and liquid Fresubin consumption, the fraction of CeMHtr2a cells displaying a stable correlation was significantly larger than the fraction of cells that changed their activity (58% versus 42%) (Fig. 6l). Instead, the fraction of CeMSst cells showing a stable correlation was comparable to the fractions of cells that changed their activity (Supplementary Fig. 6j). In summary, at the population level, the majority of CeMHtr2a and CeMSst neurons increased their activities during reward consumption and their activity dynamics contributed to and decoded specific behavioral features. Depending on the physical attributes of the rewards, CeMHtr2a and CeMSst neurons were recruited into different ensembles with constant or variable correlated activity.
CeMHtr2a and CeMSst neurons respond differently to stimuli of opposite valence
To investigate what fractions of CeMHtr2a and CeMSst neurons respond specifically to one class of stimuli (“specializers”) or exhibit a general response to a broad range of stimuli even of opposite valence (“generalizers”), we exposed the same cohorts of mice expressing GCaMP6m (Supplementary Fig. 7a) to three different aqueous solutions: water, saccharin, and the bitter tastant quinine. During each 10-min session, the stimulus was orally administered to water-deprived mice at minutes 1 and 6 by an experimenter who the mice were well accustomed to. During the remaining time, mice were allowed to freely behave (Fig. 7a). We recorded from an average of 107 CeMHtr2a and 101 CeMSst neurons and an average of 71 neurons was longitudinally detected in different sessions (Supplementary Fig. 7b, c). Many CeMHtr2a and CeMSst neurons showed an increase in calcium responses to all three stimuli during the stimulus exposure periods (Fig. 7b). When analyzing the average population activity across multiple 10-min sessions, we found that the switch from water to saccharin, and water to quinine-added water, did not significantly change activity (although the latter was close to significance). Interestingly, we observed a significant decrease in population activity when switching from saccharin to quinine (Fig. 7c). These results suggest that the switch from sweet to bitter taste quenched the activities of both subpopulations. We applied the CEBRA Hybrid model on the recorded neuronal activities of both CeMHtr2a and CeMSst neurons during the water, saccharin, and quinine behaviors. The observable structures suggested that these behaviors contributed significantly to the alterations in neuronal activity (Fig. 7d). The embeddings of both neuron populations could accurately decode positive and negative stimuli with higher accuracies (97–100%) and lower OOB errors compared to the shuffled data (71–73%) (Fig. 7e; Supplementary Fig. 7e).
a Behavioral paradigm. b Representative trace from a CeMHtr2a neuron showing increased neuronal activity during the 1-min consumption bouts (gray color). c Average z-score comparisons of the activities of the same CeMHtr2a and CeMSst neurons recorded in the three different conditions (two-tailed Wilcoxon matched-pairs signed-rank test. CeMHtr2a: water-saccharin, p = 0.9477, n = 80; saccharin-quinine, p = 0.0175, n = 71; water-quinine, p = 0.0714, n = 67. CeMSst: water-saccharin, p = 0.1836, n = 69; water-quinine, p = 0.6808, n = 63; saccharin-quinine, paired two-tailed t test p = 0.0067, t = 2.788, n = 78). d CEBRA-generated embeddings of CeMHtr2a and CeMSst neurons in the three conditions. e Comparison of behavioral decoding accuracy using RF across animals for Htr2a (blue) and Sst (green) neurons (water, saccharin, quinine: two-tailed Wilcoxon matched-pairs signed-rank test p < 0.0001). (Prediction scores of accuracy of behavior compared to shuffled data. Htr2a: Actual-Shuffled n = 16. Sst: Actual-Shuffled: n = 57). Chord diagrams and bar graphs showing the stable (“generalizers”) or unstable correlation (“specializers”) of the same CeMHtr2a (f) and CeMSst neurons (g) when switching from saccharin to quinine. Specializers in gray (CeMHtr2a unpaired two-tailed t test p = 0.0332, t = 2.569, n = 5 mice; CeMSst p = 0.0087, t = 3.047, n = 8 mice). h Percentages of generalizers (left) and specializers (right) within the CeMHtr2a and CeMSst populations (Generalizer: unpaired two-tailed t test p = 0.0259, t = 2.573. Specializer: p = 0.0261, t = 2.570). (Htr2a: n = 5, Sst: n = 8 mice). i Photoactivation of CeMHtr2a (blue) and CeMSst neurons (green) promotes 10 mM quinine intake compared to controls (CeMHtr2a: Mann-Whitney two-tailed test p = 0.0007, U = 3.500. CeMSst: p = 0.0016, U = 5). (CeMHtr2a: n = 8 Ctrl, n = 9 ChR2; CeMSst: n = 8 Ctrl, n = 9 ChR2 mice). j Same as in (i), except for 100 μM quinine (CeMHtr2a: Mann-Whitney two-tailed test p = 0.0003, U = 2. CeMSst: p = 0.0151, U = 11). Group sizes as in (i). CeMHtr2a::ChR2 and CeMSst::ChR2 mice drink larger amounts of 100 µM compared to 10 mM quinine (CeMHtr2a main effect quinine: Two-way ANOVA, F(1,15) = 14.83, p = 0.0016; multiple comparison p = 0.0003. CeMSst main effect quinine: Two-way ANOVA, F(1,15) = 8.711, p = 0.0099; multiple comparison p = 0.0015). (n = 8 Ctrl, n = 9 ChR2 mice). Values = Mean ± SEM.
Next, we compared the activity during the stimulus exposure episodes with the times in-between and asked which cells were positively or negatively correlated, or showed an activity that was not significantly correlated with stimulus presentation (neutral). We then asked how many cells could be qualified as generalizers showing a stable correlation (positive–positive, negative–negative) between oppositely valenced stimuli, and how many cells would be specializers that switched their correlation. For CeMHtr2a neurons, during the switch from saccharin to quinine, the fraction of specializers was significantly larger than the fraction of generalizers (60% versus 40%). Conversely, for CeMSst cells the pattern was opposite, with generalizers outnumbering specializers (64% versus 36%) (Fig. 7f–h,). Other comparisons did not yield significant differences between generalizers and specializers (Supplementary Fig. 7f). In summary, these results suggest that the activities of CeMHtr2a and CeMSst neurons contribute to the detection of stimuli of opposite valence, and that the CeMHtr2a population contains more cells that specialize in encoding valence-specific stimuli than CeMSst neurons.
To disentangle between activation of a fluid intake motor program (as suggested by the photoactivation experiments) and valence detection, we next asked if photoactivation of CeMHtr2a or CeMSst neurons would be sufficient to induce consumption of quinine adulterated water. Neurons were photoactivated during 30 min in which water-deprived mice were exposed to a 10 mM quinine solution. While photoactivated control mice avoided the bitter solution, activation of CeMHtr2a and CeMSst neurons stimulated quinine consumption (Fig. 7i). The same mice were tested with a subtler quinine solution (100 µM) under similar conditions and were found to consume more fluid compared to controls (Fig. 7j). Comparing the intake of the two quinine concentrations by the same mice, we found that ChR2-expressing mice drank significantly more of the lower-concentrated solution. These findings suggest that photoactivation of CeMHtr2a and CeMSst neurons stimulates a fluid intake motor program, but that the mice are still able to distinguish varying concentrations of unpleasant taste.
Appetitive CeM neurons are inhibited by anorexigenic CeAPKCδ neurons
Next, we asked how the activities of appetitive CeMHtr2a and CeMSst may be regulated. Previous studies had suggested that putative appetitive CeA neurons may be under inhibitory control of anorexigenic CeAPKCδ neurons6 and that CeAHtr2a neurons engage in reciprocal inhibitory connections with CeAPKCδ neurons5. This raised the possibility that CeMHtr2a and CeMSst neurons may also receive direct inhibitory input from CeAPKCδ neurons that reside in the CeL/C subregion. We hypothesized that inhibition of CeAPKCδ neurons would disinhibit and thereby activate appetitive CeMHtr2a and CeMSst neurons, whereas activation of CeAPKCδ neurons would inhibit them. We first confirmed that photoactivation of PKCδ cells suppressed water consumption compared to photostimulated control mice (Fig. 8a–d). In a 10-min ON/OFF stimulation protocol (similar to Fig. 2, but starting with Light ON), the control group consumed most of the water during the initial 10 min, then gradually decreased consumption as the mice became satiated, regardless of the light phase. In contrast, CeAPKCδ neurons consumed water only during the Light OFF phases (Fig. 8d). Conversely, photoinhibition of CeAPKCδ neurons using Cre-dependent Halorhodopsin (Fig. 8e, f) resulted in increased water intake compared to photostimulated control mice (Fig. 8g). Notably, anxiety did not appear to affect the behavioral outcomes, as the mice subjected to photoactivation or inhibition of CeAPKCδ neurons spent a comparable amount of time in the center-zone during the open-field test as the control group (Fig. 8h, i).
a Viruses injected and optic fiber placement in the CeA (modified from Allen Mouse Brain Atlas, mouse.brain-map.org). b EYFP and ChR2 expression in the CeL of PKCδ-Cre mice. All mice used for behavior showed similar results. c Water intake during 30 min CeAPKCδ photoactivation in water-deprived mice (two-tailed Wilcoxon signed-rank test p = 0.0020). (n = 10 mice each). d Water intake during photoactivation of CeAPKCδ neurons in a 10 min Light ON/OFF behavioral paradigm (Light ON, light blue shading) by water-deprived mice (0–10 min, Mann-Whitney two-tailed U test p < 0.0001, U = 0; 10–20 min, p = 0.0004, U = 5; 20–30 min, two-tailed Wilcoxon signed-rank test p = 0.0078; 30–40 min, Mann-Whitney two-tailed U test p = 0.0002, U = 4; 40–50 min two-tailed Wilcoxon signed-rank test p = 0.0312; 50–60 min Mann-Whitney U test p = 0.0011, U = 9). (n = 10 mice each). e Same as in (a) except for eNpHR3.0 expression (modified from Allen Mouse Brain Atlas, mouse.brain-map.org). f Same as in (b) except for mCherry and eNpHR3.0 expression. g Water intake during 30 min photoinhibition of CeAPKCδ neurons by water-deprived mice (unpaired two-tailed t test p = 0.0282, t = 2.365). (n = 11 mice each). Time in the center during an OF test by photoactivated PKCδ-Cre::ChR2 (h) (Mann-Whitney two-tailed U test p = 0.4359, U = 39, n = 10 mice each) and by photoinhibited PKCδ-Cre::eNpHR3.0 (i) (unpaired two-tailed t test p = 0.2719, t = 1.133, n = 11 mice each). j PKCδ-Flp transgene. NLSFLPo-pA, optimized FLP with NLS and polyA recognition sequence. mCherry (red) and PKCδ (green) expression in the CeA of PKCδ-Flp;FPDi mice. Fraction of mCherry+ among PKCδ cells: 88.7 ± 1.0% (n = 3 brains, 3 sections per brain). k Intersectional strategy to map PKCδ-CeM connectivity. l Example trace of a CeAPKCδ neuron photostimulated with 1, 10, and 20 Hz in slices. m Representative example of photostimulated (20 Hz) CeAPKCδ neuron suppressing current-injection-induced firing of a CeMSst neuron. n Representative traces of induced inhibitory postsynaptic current (IPSC) in CeMHtr2a (left) and CeMSst (right) cells after photoactivation of CeAPKCδ neurons. o Quantification of IPSC amplitudes of recorded CeMHtr2a and CeMSst neurons (n = 22 for CeMHtr2a, n = 18 for CeMSst neurons). Values = Mean ± SEM. Scale bar: 115 μm.
To demonstrate a functional connection between CeAPKCδ and the appetitive CeM subpopulations, we generated the mouse line PKCδ-Flp, that expresses the Flp recombinase specifically under the control of the PKCδ promoter (Fig. 8j). We validated the expression of the Flp recombinase by crossing the PKCδ-Flp mouse line with a Flp-dependent reporter mouse and quantified the numbers of reporter-positive cells versus PKCδ immunostaining. The results revealed that 89% of the PKCδ immunopositive cells colocalized with the reporter (Fig. 8j). Using an intersectional approach, we crossed PKCδ-Flp mice with Htr2a-Cre mice also carrying a Cre-dependent tdTomato reporter (Ai9) to later visualize CeMHtr2a neurons in slices. Similar crosses were done with Sst-Cre mice (Fig. 8k). We then injected a Flp-dependent ChR2-EYFP AAV into the CeA to photoactivate CeAPKCδ neurons in slices. With this design, it was possible to patch and record from CeM tdTomato-positive neurons while photoactivating CeAPKCδ neurons in the CeL (Fig. 8k). These results showed that photoactivation of CeAPKCδ neurons suppressed current-induced firing of cells in the CeM (Fig. 8l, m). Additionally, we could record inhibitory postsynaptic currents from CeMHtr2a and CeMSst neurons, suggesting evidence for a monosynaptic connection from CeAPKCδ to both CeM subpopulations (Fig. 8n, o). Together, these findings demonstrate that CeAPKCδ neurons suppress water intake and inhibit the activities of CeMHtr2a and CeMSst neurons in slices, consistent with a model in which the activities of CeMHtr2a and CeMSst neurons are under inhibitory control of CeAPKCδ neurons in vivo.
Htr2a and Sst neurons send projections to brain regions associated with reward processing
After having identified one of the possible mechanisms regulating the activity of Htr2a and Sst neurons in the CeM, we proceeded to anatomically map the long-range outputs of these neurons. To identify the major output targets of Htr2a and Sst neurons in the CeL and CeM, we selectively expressed either Con/Fon or Con/Foff YFP viruses in Htr2a/Sst-Cre::Wfs1-FlpoER mice. To determine the relative strength of the projections, we calculated the integrated fluorescence intensities in the output region normalized to background and injection sites. Our analysis revealed that for Htr2a neurons, the CeM fraction projected to a larger number of outputs compared to the CeL fraction (19 versus 8), whereas for Sst neurons, CeM and CeL fractions projected to similar numbers of outputs (20 versus 16) (Supplementary Figs. 8 and 9). A number of brain regions received strong projections from both Htr2a and Sst CeL and CeM fractions, including the bed nucleus of the stria terminalis (BNST), the lateral vestibular nucleus (LAV), the lateral and medial parabrachial nucleus (LPBN, MPBN), and the midbrain reticular nucleus (MRN) (Fig. 9a–d). The interstitial nucleus of the posterior limb of the anterior commissure (IPAC) and the substantia innominata (SI) received stronger projections from the CeM compared to the CeL fractions. Few brain regions received enriched projections from CeMSst neurons, including the lateral and medial geniculate complex (LG, MG) and the ventral posterolateral/medial nucleus of the thalamus (VPL/M) (Fig. 9a–d; Supplementary Figs. 8 and 9). Several of these regions are known to be involved in rewarding and consummatory behaviors.
Brain regions that receive projections from CeMHtr2a (a), CeLHtr2a neurons (b), CeMSst (c), and CeLSst neurons (d), and corresponding box-plots with quantification of fluorescence intensities (n = 3 mice per condition). Values = Median (Center) ± Min/Max (whiskers). 25–75 percentile (box). Scale bar: 500 μm. Abbreviations: AC anterior commissure, BNST bed nucleus of the stria terminalis, CeL central amygdala lateral part, CeM central amygdala medial part, GPe globus pallidus, external segment, IPAC interstitial nucleus of the anterior commissure, LAV lateral vestibular nucleus, MG medial geniculate complex, MRN midbrain reticular nucleus, L/MPBN lateral/medial parabrachial nucleus, PSV principal sensory nucleus of the trigeminal, SI Substantia innominata, SN substantia nigra, VPL ventral lateral nucleus of the thalamus. e Bilateral CeA viral injection and bilateral optic fiber placement above the PBN (modified from Allen Mouse Brain Atlas, mouse.brain-map.org). Water intake during photoactivation of PBN projections of CeMHtr2a (f) or CeMSst (g) neurons by normally hydrated mice compared to controls (CeMHtr2a: Mann-Whitney two-tailed U test p = 0.0021, U = 22.50 and CeMSst: p = 0.0008, U = 13). (CeMHtr2a to PBN: n = 14 Ctrl, n = 8 ChR2. CeMSst to PBN: n = 11 Ctrl, n = 10 ChR2 mice). Food intake during photoactivation of PBN projections of CeMHtr2a (h) or CeMSst (i) neurons by satiated mice compared to controls (CeMHtr2a: Mann-Whitney two-tailed U test p = 0.0061, U = 23 and CeMSst: p = 0.9265, U = 63.50). (CeMHtr2a to PBN: n = 12 Ctrl, n = 10 ChR2. CeMSst to PBN: n = 13 Ctrl, n = 10 ChR2 mice). Preference for the light-paired chamber in the RTPP task when PBN projections of CeMHtr2a (j) or CeMSst (k) neurons are activated, compared to controls (CeMHtr2a: unpaired two-tailed t test p = 0.0023, t = 3.522 and CeMSst: p < 0.0001 t = 5.949). (CeMHtr2a to PBN: n = 12 Ctrl, n = 9 ChR2. CeMSst to PBN: n = 11 Ctrl, n = 7 ChR2 mice). Values = Mean ± SEM. l. The CeA appetitive microcircuit. All CeL and CeM appetitive neurons encode positive valence and form long-range projections. CeMHtr2a and CeMSst neurons promote water intake, CeMHtr2a neurons promote feeding. They are inhibited by anorexigenic CeAPKCδ neurons. Activation of PBN projections of CeMHtr2a or CeMSst neurons promotes food and water intake.
The appetitive functions of CeMHtr2a and CeMSst neurons are mediated by projections to the PBN
Based on the known functions of the PBN serving as a hub for sensory information relevant to food and water intake, receiving inputs from interoceptive and exteroceptive sources38,39,40,41,42,43,44, and driving aversive emotional behaviors, we hypothesized that inhibition of PBN neurons by CeMHtr2a or CeMSst neurons may promote drinking and/or feeding behavior. To explore the functions of these neuronal projections, we injected a Con/Foff ChR2 virus, or similar control virus, bilaterally into the CeA of Htr2a-Cre::Wfs1-FlpoER or Sst-Cre::Wfs1-FlpoER mice and placed optic fibers bilaterally above the PBN (Fig. 9e, Supplementary Fig. 10a, b). Photoactivation of the presynaptic terminals in the PBN of CeMHtr2a or CeMSst neurons led to increased drinking in both water-deprived and hydrated mice during both a 30 min and a 10 min Light ON/OFF stimulation protocol (Fig. 9f, g, Supplementary Fig. 10c–h.). Furthermore, photoactivation of the CeMHtr2a → PBN projectors stimulated feeding behavior, whereas, as expected, no effect was observed for the CeMSst → PBN projectors (Fig. 9h, i). Moreover, both CeMHtr2a → PBN and CeMSst → PBN projectors promoted rewarding behavior in real-time place preference (Fig. 9j, k) and conditioned flavor preference assays (Supplementary Fig. 10i–l). No changes in locomotion or anxiety were observed in the open-field test (Supplementary Fig. 10m, n). These findings suggest that CeMHtr2a and CeMSst neurons promote appetitive and reward behavior through inhibition of PBN neurons.
Discussion
In this report, we have used an intersectional genetics approach to independently target four putative appetitive neuron subpopulations in the central amygdala: two in the CeL and two in the CeM. We found that neurons mediating water and/or food consumption are confined to the CeM. Separate CeM subpopulations exist for water only (CeMSst), and water or food consumption (CeMHtr2a). All four subpopulations are intrinsically positively reinforcing in a conditioned flavor preference assay. Through in vivo calcium imaging we observed that the majority of CeMHtr2a and CeMSst neurons increased their activity during reward consumption and that their activity dynamics contributed to and decoded specific behavioral features. Depending on the type of rewards, CeMHtr2a and CeMSst neurons were recruited into different ensembles with constant or variable correlated activity. Calcium imaging further suggests that the activities of CeMHtr2a and CeMSst neurons contribute to the detection of stimuli of opposite valence, and that the CeMHtr2a population contains more cells that specialize in encoding valence-specific stimuli than CeMSst neurons. At the microcircuit level, the activity of appetitive CeM neurons is controlled by inhibitory signals from CeAPKCδ neurons and, in turn, appetitive CeM neurons form long-range inhibitory projections to the PBN to promote appetitive and reward behavior (Fig. 9l). In summary, this study provides a comprehensive functional characterization of molecularly- and anatomically-defined CeA neurons and their roles in appetitive behaviors.
Intersectional genetics using two recombinases has become a valuable tool to address specific neuron subtypes within a larger population of neurons, for example, distinct subtypes of midbrain dopaminergic neurons or hypothalamic POMC neurons45,46. This method has also been useful to manipulate specific neurons within an anatomically well-defined area, for example, specific Cre+ neuron populations in the spinal cord, and to assess the functional consequences without interference by Cre recombination in the brain47. Here, we have used the Wfs1-FlpoER mouse line that expresses the optimized and tamoxifen-inducible FlpoER recombinase in the CeL subdivision of the CeA, to a much higher degree (17-fold) than in cells of the CeM. In combination with a specific Cre line and a Con/Fon Boolean reporter virus, we could target between 4 and 10 times more Cre+ cells in the CeL than CeM. Conversely, with a Con/Foff virus, we could target between 2.3 and 2.7 times more Cre+ cells in the CeM than CeL. These results indicate that the system is effective at producing an enrichment of CeL versus CeM subpopulations. The inferior performance of the Con/Foff virus may have resulted from incomplete coverage of Wfs1-Flp expression in CeL neurons. Cre-positive CeL cells that do not co-express Flp would express the Con/Foff reporter and thereby lower the CeM:CeL ratio. As for most biological systems, the expression in the CeL versus CeM subpopulations is not black-and-white. The strength of the system relies on the combination of the INTRSECT viruses with photoactivation and -inhibition experiments.
The CeL is largely composed of three cell populations: CeLPKCδ neurons that inhibit food or water consumption (this study and6,7,48,49,50), and Sst+ neurons that can be further subdivided into CeLSst and CeLNts/Tac2 cells7,13,22. Both Sst+ populations in CeL heavily overlap with Htr2a-Cre expressing cells13. Here, by targeting Sst-Cre- or Htr2a-Cre-positive cells in the CeL, we obtained no evidence that they could promote water or food consumption. This was a surprising finding in light of earlier reports showing that Sst+, Nts+, Pnoc+, and Htr2a+ neurons promoted water, palatable fluid, and/or palatable food intake5,7,23,24. However, these reports did not distinguish between CeL and CeM subpopulations, raising the possibility that it was indeed the CeM subpopulations that drove the behavior. The study by Kim et al.7, attempted to target the anatomical subregions with stereotaxic viral injections and reported that both CeL and CeM subpopulations of Sst+ and Nts/Tac2+ neurons promoted water intake. This result is contrary to ours, and may in part be due to the different techniques employed. Further experiments would be necessary to solve this discrepancy.
Contrary to the CeL, the Sst+ and Htr2a-Cre+ subpopulations in the CeM are largely separate populations13. Here, we show that CeMSst neurons promote water, but not solid food intake, whereas CeMHtr2a neurons promote water and food intake. These findings are consistent with and extend earlier observations: Nts+ neurons (a subpopulation of Sst neurons) promote ethanol and palatable fluid, but not solid food, consumption24; photoactivation of Tac2+ or CRH+ cells (subpopulations of Sst neurons) has no positive effect on feeding6; Pnoc+ neurons (heavily overlapping with Htr2a-Cre-expressing neurons) promote palatable food consumption23, photoactivation of NPY neurons, located in the CeM and overlapping with Htr2a neurons, increases food intake51.
The optogenetic experiments are supported by in vivo calcium imaging data which indicate that the majority of CeMHtr2a and CeMSst neurons increased their activity during consumption of water and food rewards. CEBRA analysis revealed that their activity dynamics contributed to and decoded specific behavioral features. Depending on the type of rewards, CeMHtr2a and CeMSst neurons were recruited into different ensembles whose activities correlated positively or negatively with reward consumption. The highest positive correlation of CeMHtr2a neurons was with feeding (59%), consistent with their observed function in food consumption. When switching between rewards of different physical attributes (e.g., solid food and liquid Fresubin), CeMHtr2a neurons more often displayed a stable correlation, while CeMSst neurons more often changed their activity. These results are consistent with previously suggested models11,12 in which CeASst neurons participate in discriminating between stimuli that differ in their sensory/physical properties, such as taste and texture. In contrast to CeMSst neurons, CeMHtr2a neurons may encode a broader range of information, such as the innately affective properties of the stimulus and the animal’s internal hunger state. CeMHtr2a cells are activated by fasting and the hunger hormone ghrelin13 and here, we found that a higher percentage of CeMHtr2a cells were positively correlated with feeding when the animals were hungry compared to when they were fed and consuming Fresubin. Hence, CeMHtr2a neuron activity is less correlated with the physical attributes of the stimulus, and more with its palatability and rewarding properties.
If the activity of CeMSst neurons increases during water licking and feeding bouts, why is ectopic activation of these neurons sufficient to promote drinking, but not food consumption? Previously, it was shown that the activity of Sst+ neurons (in the entire CeA) arose later than the animal’s licking responses following water delivery, suggesting that the activity of these neurons did not promote licking12. Instead, Sst+ neurons may drive water consumption by conveying stimulus-specific signals to downstream reward centers. Solid food consumption involves additional aspects such as handling and biting the food, and these aspects may only be driven by CeMHtr2a, but not CeMSst neurons. Further work will be necessary to test this hypothesis.
At the population level, Sst+ CeA neurons were previously shown to encode a range of appetitive and aversive stimuli. Many individual Sst+ neurons displayed high selectivity to only one class of stimuli (“specializers”), some responded to multiple stimuli, sometimes of opposite valence (“generalizers”)12. Similar heterogeneity may be present in other CeA populations, such as Nts+ neurons which promote the intake of palatable fluids, but not solid food24. Here, we also recorded the activity of individual CeM neurons when animals experienced a switch between an appetitive and an aversive liquid. For CeMHtr2a neurons, the fraction of specializers was significantly larger than the fraction of generalizers when switching from saccharin to quinine solution. Interestingly, for the same switch in stimuli, the pattern was opposite for CeMSst cells, with generalizers outnumbering specializers. We conclude that the CeMHtr2a population contains more cells that innately specialize in encoding valence-specific stimuli than the CeMSst population. In other words, for CeMHtr2a neurons that may integrate physical and rewarding properties of the stimulus with the animal’s hunger state, the switch from saccharin to quinine represents an important valence switch that causes a change in activity for most neurons. For CeMSst cells that mainly respond to stimuli that differ in their sensory/physical properties, a switch between a sweet and a bitter liquid represents a minor difference in physical attributes that did not cause a change in activity for most neurons.
We also showed that photoactivation of CeMHtr2a or CeMSst neurons was sufficient to induce consumption of quinine adulterated water and the amount consumed negatively correlated with the dose of quinine. These findings suggest that photoactivation of CeMHtr2a and CeMSst neurons stimulates a fluid intake motor program, but that the mice are still able to distinguish varying concentrations of unpleasant taste. We conclude that photoactivation of the neurons has two effects that contribute to fluid consumption: First, the activation of a fluid intake motor program that drives drinking independent of internal state and despite strongly aversive taste, and second, the generation of a rewarding effect that counterbalances the bitter taste. The stronger the bitter taste, the more it cancels out the rewarding effects and the less is consumed by the mice.
Previously, the activities of Sst+ and Htr2a-Cre-expressing neurons (of the entire CeA) were shown to be intrinsically rewarding in RTPP assays, and intrinsically reinforcing in intracranial self-stimulation and reward learning assays5,7,52. Here, we show that both CeM subtypes drive RTPP and conditioned flavor preference. It is likely that this activity contributes to the promotion of water or food intake. The CeL subtypes of Sst+ and Htr2a-Cre-expressing neurons also displayed modest reinforcing activity in the conditioned flavor preference assay, but perhaps not in RTPP. These observations are in line with the notion that Sst+ neurons are required for learning and that Sst+ neurons projecting to substantia nigra (SN) dopaminergic neurons participate specifically in reward learning12.
The appetitive inputs that lead to activation of CeM subtypes are not well understood. Brain regions controlling water intake include the anterior cingulate cortex and their projections to the amygdala complex53 and the peri-locus coeruleus54. Food-related inputs may come from insular cortex21,55, PBN38,42, the arcuate nucleus5,56 and the parasubthalamic nucleus5,57, from fasting, and the hunger hormone ghrelin13. Here, we have shown that both CeM subtypes are under inhibitory control of CeAPKCδ neurons residing in the CeL. When animals reach satiety during consumption of water or food, the behavior is terminated by activation of CGRP+ PBN neurons38,41 that project to the CeA and activate PKCδ neurons. Conversely, when animals are thirsty or hungry, CeAPKCδ neurons may become inhibited, either through the local CeL inhibitory network, or by long-range inhibitory inputs to CeAPKCδ neurons, and this will disinhibit CeMHtr2a and CeMSst neurons favoring the expression of appetitive behavior.
A number of brain regions received strong projections from both Htr2a and Sst CeL and CeM fractions. Among them, the BNST plays a central role in reward-related behaviors58, while also influencing feeding through its projections to the lateral hypothalamus (LH)59,60. The parabrachial nucleus is a sensory relay receiving an array of interoceptive and exteroceptive inputs relevant to taste and ingestive behavior, pain, and multiple aspects of autonomic control38,40,44,61,62. Brain regions that receive stronger projections from CeM than CeL neurons include the IPAC, located within the extended amygdala, and known to regulate energy homeostasis63 and the SI, a basal forebrain structure involved in reinforcement learning64. Few brain regions received enriched projections from CeMSst neurons. Among them, the lateral and medial geniculate complex (LG, MG), thalamic nodes involved in defensive behaviors, and the ventral posterolateral/medial nucleus of the thalamus (VPL/M), a relay for somatosensory signals65,66,67,68,69.
The PBN is an interesting output region because it receives projections from CeMHtr2a neurons driving food consumption, from CeMSst neurons driving water consumption, and from CeMDlk1 neurons suppressing food intake during nausea70. It is likely that CeMHtr2a → PBN and CeMSst → PBN projectors target different microcircuits in the PBN, since their optogenetic activation elicited different behaviors. Anorexigenic CeMDlk1 → PBN projectors may interfere with the appetitive CeM→PBN projectors, for example, through a presynaptic inhibition mechanism. Future work is needed to work out the information flow from the CeM to the PBN.
In conclusion, the present manuscript provides a detailed functional characterization of molecularly- and anatomically-defined appetitive CeA subpopulations. Our findings indicate that neurons driving food or water consumption are confined to the CeM and that separate CeM subpopulations regulate water only (CeMSst), versus water or food consumption (CeMHtr2a). The response properties of these CeM neurons show interesting differences regarding reward value and physical attributes of the stimuli. In the future, further characterization of these circuits, including modulation by neuropeptides and comparative evolutionary studies in healthy and diseased subjects may provide insights into the etiology of eating and drinking disorders and may help to develop therapeutic strategies to combat these common problems.
Methods
Animals
Experiments were always performed using adult mice (>12 weeks). Mice are group-housed under standard laboratory conditions and maintained under a 12-h light/12-h dark cycle with food and water ad libitum. The Htr2a-Cre BAC transgenic line (stock Tg(Htr2a-Cre)KM208Gsat/Mmucd) was imported from the Mutant Mouse Regional Resource Center 482 (https://www.mmrrc.org/). SOM-IRES-cre (SSTtm2.1(cre)Zjh/J) mice were acquired from the Jackson Laboratory. Ai9lsl−TdTomato (B6.Cg- Gt(ROSA) 26Sortm9(CAG- tdTomato)Hze/J)71, FPDI (B6;129S6Gt(ROSA)26Sortm9 (CAG-mCherry, -CHRM4*)Dym)35 mouse lines were as described previously. All mice were backcrossed into a C57BL/6NRj background (Janvier Labs—http://www.janvier-labs.com). Both male and female mice were used and all the experiments were performed following regulations from the government of Upper Bavaria.
Generation of Wfs1-FlpoER transgenic mice
For the generation of Wfs1-FlpoER mice, a BAC homologous recombination method was used. The BAC clone RP23-405O19 (CHORI) was targeted with a FLPoERT2 expression cassette72 and a WPRE sequence followed by the bovine growth hormone polyadenylation signal (pA) and a kanamycin resistance cassette. The BAC homologous recombination cassette was assembled in the pcDNA3.1 (+) vector (Invitrogen) as follows: Homology arms A and B were designed to flank mouse Wfs1 exon 2. A construct containing the homology arm A and the NLSFlpoERT2WPREpA sequences was synthetized by Eurofins and cloned into the pUC57 vector (GenScript). Homology arm B was synthetized by PCR using the following primer sets: 5′-CGATATCAACTCAGGCACC-3′ (forward primer for arm B), 5′-AATCTCGAGCAGGGACACTG-3′ (reverse primer for arm B). First, pCDNA3.1 (+) was digested with NheI/AflII to insert the homologous arm A – NLSFlpoERT2WPREpA fragment into this site. Second, the kanamycin resistance cassette (Gene Bridges GmbH) was cloned into the AflII/EcoRV site before the arm B. Then, the homologous arm B was inserted into the EcoRV/XhoI site. This targeting vector was digested with NheI/XhoI followed by purification of the insert on a 0.7% agarose gel. Homologous recombinant BACs were obtained using established methods73,74, screened by PCR, and verified by Southern blotting. Modified BAC DNA was prepared using the large construct DNA purification kit—NucleoBond® Xtra BAC (Macherey-Nagel), linearized with PmeI and purified through a Sepharose™ separation column75. BAC DNA (2.2 ng/μl) was injected into pronuclei of fertilized oocytes of C57BL/6 mice. BAC transgenic mice were identified by PCR using the primers 5′ GCTCTATTCAGGACATTTTCACATCTCTAC 3′ and 5′ CCTCTCGAATCTCTCCACGAAC 3′. The Wfs-FlpoER transgene was inserted in chromosome 15 between positions 11,537,026 and 11,537,028 (mm10 Mus musculus reference genome). A small 6 bp genomic duplication was found at the integration site. According to RefSeq, there are no genes annotated in this region.
Generation of PKCδ-Flpo transgenic mice
A recombineering protocol was used to insert an NLSFLPo-pA expression cassette76 into the ATG start site of the PKCdelta locus on the BAC clone RP23-283B12 (CHORI). The wild-type loxP site present in the RP23 BAC backbone was replaced by a piggyBAC-ampR cassette. Plasmid construction and BAC modification was done by Gene Bridges GmbH. BAC DNA for pronuclear injection was prepared using the large construct DNA purification kit—NucleoBond® Xtra BAC (Macherey-Nagel), linearized with PI-SceI and purified over a home-made Sepharose CL4B column (Johansson et al.75). Fractions were analyzed by pulsed-field gel electrophoresis to identify the sample with the highest concentration of linearized BAC DNA and lowest concentration of vector DNA. Linearized BAC DNA was injected at a concentration of 3.65 ng/μl into pronuclei of fertilized oocytes of C57BL/6 mice. BAC transgenic mice were identified by PCR using the primers 5′ AAACTGCATCACCTTCTCACATCTCC 3′ and 5′ CTCTCGAATCTCTCCACGAACTGC 3′. The Pkcδ-Flpo transgene was inserted in chromosome 15 between positions 21,426,977 and 21,427,824 (mm10 Mus musculus reference genome), leading to an 846 bp genomic deletion of the host genome. According to RefSeq, intron 3 of Cdh12 is annotated in the deleted region.
Viral constructs
The following AAV viruses were purchased from the University of North Carolina Vector Core (https://www.med.unc.edu/genetherapy/vectorcore): AAV5-Ef1a-DIO-eNpHR3.0-mCherry, AAV5-Ef1a-DIO-mCherry, AAV5-Ef1a-DIO-hChR2(H134R)-EYFP-WPRE-pA, AAV5-Ef1a-DIO-EYFP-WPRE-pA, AAV5-hSyn-Con/Fon-hChR2(H134R)-EYFP-WPRE, AAV5-hSyn-Con/Fon-EYFP-WPRE, AAV5-hSyn-Con/Foff-hChR2(H134R)-EYFP-WPRE, AAV5-hSyn-Con/Foff-EYFP-WPRE, AAV5-EF1a-fDIO-hChR2(H134R)-EYFP-WPRE.
AAV8-nEF-Con/Foff iC++-EYFP, AAV8-nEF-Con/Foff-EYFP and AAV8-EF1a-Con/Foff-GCaMP6m viruses were generated as described32.
Viral injections
Mice were anaesthetized using isoflurane (Cp-pharma) and placed on a heating pad on a stereotaxic frame (Model 1900—Kopf Instruments). Carprofen (Rimadyl—Zoetis) (20 mg/kg body weight) was given via subcutaneous injection. Mice were bilaterally (or unilaterally for calcium imaging experiments) injected using glass pipettes (#708707, BLAUBRAND intraMARK) with 0.3 µl of virus in the CeA by using the following coordinates calculated with respect to bregma: for the CeA and CeL: −1.20 mm anteroposterior, ±2.87 mm lateral, −4.65 to −4.72 mm ventral; for the CeM −1.155 mm anteroposterior, ±2.87 mm lateral, −4.65 to −4.72 mm ventral. The following stereotaxic coordinates were used for the PBN: −5.2 mm anteroposterior, ±1.4 mm lateral, −3.85 mm ventral. Virus was allowed to be expressed for a minimum duration of 3 weeks before histology or behavioral paradigms. For animals not undergoing implant surgery, the incision was sutured.
Optic fiber implants
Mice used in optogenetic experiments were implanted with optic fibers (200-µm core, 0.22 NA, 1.25-mm ferrule—Thorlabs) above the CeA (−4.35 mm ventral from bregma) or the PBN (−3.6 mm ventral from bregma) immediately after viral injection. The skull was first protected with a layer of histo glue (Histoacryl, Braun), the fibers were then fixed to the skull using UV light-curable glue (Loctite AA3491—Henkel), and the exposed skull was covered with dental acrylic (Paladur–Heraeus).
GRIN lens implantation and baseplate fixation
Three weeks after GCaMP6m viral injection in the CeA, mice were implanted with a gradient-index (GRIN) lens. At the same coordinates of the injection, a small craniotomy was made and a 20 G needle was slowly lowered into the brain to clear the path for the lens to a depth of −4.5 mm from bregma. After retraction of the needle, a GRIN lens (ProView lens; diameter, 0.5 mm; length, ~8.4 mm, Inscopix) was slowly implanted above the CeA and then fixed to the skull using UV light-curable glue (Loctite AA3491—Henkel). The skull was first protected with histo glue (Histoacryl, Braun), and the implant fixed with dental acrylic (Paladur–Heraeus). 4–8 weeks after GRIN lens implantation, mice were “baseplated” under anesthesia. Briefly, in the stereotaxic setup, a baseplate (BPL-2; Inscopix) was positioned above the GRIN lens, adjusting the distance and the focal plane until the neurons were visible. The baseplate was fixed using C&B Metabond (Parkell). A baseplate cap (BCP-2, Inscopix) was left in place to protect the lens.
Tamoxifen
Tamoxifen was prepared by dissolving 20 mg tamoxifen in 100% ethanol to obtain a final concentration of 40 mg/ml. The solution was then diluted 1:1 with Kolliphor ® EL (Sigma). By heating and stirring the mixture, the ethanol evaporates. After that, 100 µl aliquots were frozen until use. Before use, the stock solution was diluted with PBS to the desired concentration and the pH value of 7.4 was confirmed. Mice were injected with ~200 μl of tamoxifen solution (200 mg per kilogram of body weight) for 3 days every other day. The mice that underwent brain viral injection received the first injection immediately after the surgery.
Behavioral assays
Mice were bilaterally tethered to optic fiber patch cables (Doric Lenses or Thorlabs) via a mating sleeve (Thorlabs). The patch cables were connected via a rotary joint (Doric Lenses) to a 473 nm or 561 nm (CNI lasers) laser. Photoactivation and photoinhibition experiments were conducted with 10–15 mW 10 ms, 473 nm light pulses at 20 Hz, using a pulser (Prizmatix) controlled by the Ethovision software XT 14 (Noldus), or 561 nm 15 mW constant light.
Drinking behavior
For water deprivation experiments, mice were water deprived and the following day trained to drink in a 32 × 35 cm plastic arena for 30 min. This training was mainly done to habituate the mice to the new setup and train them to drink from specific pipettes. For photoactivation experiments, mice were then tested the following two days in the setup with access to two pipettes of water with a sequence of 10 min laser OFF/10 min laser ON (10 min laser ON/10 min laser OFF for PKCδ mice), or simply for 30 min laser ON. The two experiments were performed in a randomized order. For experiments in normal conditions, the same protocols were used, but the mice had ad libitum water in their cage. For photoinhibition experiments, water-deprived mice had access to water for 30 min while photoinhibited the day after the training. The amount of water was manually measured.
Feeding behavior
The experiments were conducted in a 32 × 35 cm plastic arena containing two plastic cups in opposite corners, one with dustless precision pre-weighed food pellets (20 mg each) (Bio-Serv- F0071) and one empty. Experiments were conducted over 40 min sessions and the remaining food was weighed. The day before the experiment, mice were habituated to the new food by placing some of these pellets into the cage together with the normal food.
Palatable reward consumption
Mice were food deprived for 16 h and allowed to consume a palatable reward solution (Fresubin, 2 kcal/ml) for 30–45 min. The mice went back to ad libitum food and the following day were tested for 30 min for Fresubin consumption while photoinhibited.
Real-time place preference
ChR2-expressing mice and corresponding controls were allowed to freely navigate in a custom-made plexiglas two-chambered arena (50 × 25 × 25 cm) for 20 min. ChR2-expressing mice and controls received 20 Hz 473 nm photostimulation in one compartment. The experiment was repeated for two consecutive days alternating the photostimulated and neutral chambers. Preference index = percentage of time spent in the photoactivated chamber.
Conditioned flavor preference
Mice were water deprived and for two consecutive days were given the choice between two nonnutritive flavored liquids (0.3 % grape or cherry and 0.15% saccharin) for 30 min. The preferred taste was defined as the taste from the two that the mice drank more of. Conditioning was conducted over four consecutive days with two sessions per day. The least preferred taste was paired with optogenetic activation. In conditioning session one, the least preferred taste was paired with light for 15 min. In conditioning session two, the mice were presented with the more preferred taste in the absence of photostimulation. The order of the sessions was inverted each day, occurring 4–6 h apart. Conditioned flavor preference was tested the day after the final conditioning session, when the mice were presented with both tastes. Preference index = (Preferred taste)/(Preferred + least preferred taste).
Quinine consumption
Mice were water deprived and the following day trained to drink in a 32 × 35 cm plastic arena for 30 min. The day after, mice were tested in the setup with access to two pipettes containing a 10 mM or 100 μM quinine solution for 30 min. The amount of quinine was manually measured.
Open field
Mice were allowed to explore a custom-built plexiglas arena (40 × 40 × 25 cm) for 10 min. During the whole experiment, mice received photoactivation or photoinhibition.
In vivo calcium imaging of freely moving mice
All in vivo imaging experiments were conducted on freely moving mice. GCaMP6m fluorescence signals were acquired using a miniature integrated fluorescence microscope system (nVoke—Inscopix) secured in the baseplate holder before each imaging session. Mice were habituated to the miniscope procedure for 3 days before behavioral experiments for 30 min per day. Settings were kept constant within subjects and across imaging sessions. Image acquisition and behavior were synchronized using the data acquisition box of the nVoke Imaging System (Inscopix) triggered by the Ethovision XT 14 software (Noldus) through a TTL box (Noldus) connected to the USB-IO box from the Ethovision system (Noldus). For drinking experiments, mice were water deprived and trained the next day to drink in a 32 × 35 cm plastic arena for 30 min. Calcium activity was recorded the following day during a 10-min period of habituation without water and a subsequent 10-min period of exposure to water. For feeding experiments, mice were food restricted and trained the following day to consume dustless precision pre-weighed food pellets (20 mg each) (Bio-Serv- F0071) in a 32 × 35 cm plastic arena containing two plastic cups in opposite corners, one of which empty. The next day, after 10 min of recording without food, animals were exposed to food for an additional 10 min and their calcium activity was recorded. The Fresubin experiment was conducted as previously described in this manuscript. On the test day, the calcium activity of fed mice was recorded during a 10-min period of habituation followed by a 10-min period after the introduction of Fresubin. To study water/saccharin/quinine behavior, mice were water deprived and habituated the following day to water administration in the experimental setup. On the next day, the experiment was carried out in three consecutive 10-min sessions. During the first, second, and third session, water, saccharin (0.15%), and quinine (10 mM) were respectively administered at minutes 1 and 6. For imaging data processing and analysis we used IDPS (Inscopix data 721 processing software) version 1.8.0.
For the stable-unstable and generalizer-specializer analysis we calculated the percentages of neurons per animal that were maintaining a positive or negative correlation (pos–pos, neg–neg: stable or generalizer) or that were changing the correlation (neg–pos, pos–neg, neg–neutral, pos–neutral, neutral–neg, neutral–pos: unstable or specializer) between the two stimuli.
CEBRA analysis
To understand if the recorded behaviors contributed to changes in neural activity, we applied the CEBRA pipeline for feature selection. If certain behaviors account significantly for neural activity, we would have expected to see clear structures in the CEBRA embeddings compared to shuffled neural data. In brief, we chose the CEBRA Hybrid model (self-supervised) with parameters used in this study as below:
Hybrid model, model_architecture = “offset5-model-mse”, conditional = “time-delta”, hybrid = True, distance = “euclidean”, batch_size = 600–1200, learning_rate = 1e-4. Iteration = 8000.
To examine the association between CEBRA embeddings and behaviors we applied the Random Forest (RF) classifier for behavioral decoding. In brief, we fitted behavioral labels with our CEBRA embeddings, we used 70% of the data as training data and 30% of the data as test data (with data splitting in random orders). In addition, we permuted the data 1000 times randomly and tested it with the trained RF classifier. We inspected the model by checking the decoding accuracy and the out of bag (OOB) error. To prevent overfitting of the model, we decoded behavioral labels first within the same animal (Figs. 6f, g, i, j and 7e; Supplementary Figs. 6d, f and 7e), then we decoded the behavioral labels across animals (Figs. 6f, g and 7e; Supplementary Figs. 6d and 7e).
Brain-slice preparation and electrophysiological recordings
Mice were anesthetized using isoflurane and subsequently sacrificed by decapitation. The brains were then placed in a cutting solution composed of 95% oxygen and 5% carbon dioxide, with a mixture of 30 mM NaCl, 4.5 mM KCl, 1 mM MgCl2, 26 mM NaHCO3, 1.2 mM NaH2PO4, 10 mM glucose, and 194 mM sucrose. The brains were sliced to a thickness of 280 μm using a Leica VT1000S vibratome (Germany) and transferred to an artificial cerebrospinal fluid (aCSF) solution containing 124 mM NaCl, 4.5 mM KCl, 1 mM MgCl2, 26 mM NaHCO3, 1.2 mM NaH2PO4, 10 mM glucose, and 2 mM CaCl2 (310–320 mOsm), saturated with 95% O2/5% CO2 at 32 °C for 1 h before being brought to room temperature. The brain slices were then placed in a recording chamber that was continuously perfused with aCSF solution, also saturated with 95% O2/5% CO2, at a temperature of 30–32 °C.
Whole-cell patch-clamp recordings were carried out as previously described21. Patch pipettes were fabricated from filament-containing borosilicate micropipettes (World Precision Instruments) using a P-1000 micropipette puller (Sutter Instruments, Novato, CA) to achieve a resistance of 6–8 MΩ. The intracellular solution consisted of 130 mM potassium gluconate, 10 mM KCl, 2 mM MgCl2, 10 mM HEPES, 2 mM Na-ATP, 0.2 mM Na2GTP at a pH of 7.35, and an osmotic pressure of 290 mOsm. The brain slices were visualized using a fluorescence microscope equipped with IR-DIC optics (Olympus BX51). The holding potential for excitatory postsynaptic currents (EPSC) was set at −70 mV and 0 mV for inhibitory postsynaptic currents (IPSC). Data was acquired using a MultiClamp 700B amplifier, Digidata 1550 digitizer (Molecular Devices), and analyzed using Clampex 10.3 software (Molecular Devices, Sunnyvale, CA). The data was sampled at 10 kHz and filtered at 2 kHz, and further analyzed using Clampfit (Molecular Devices). A similar strategy to evaluate the connections inside the CeA was recently used for CeA-Dlk1 neurons70.
For optogenetic studies, neurons were stimulated through the use of a multi-LED array system (CoolLED) connected to an Olympus BX51 microscope.
Histology
Mice were anesthetized with ketamine/xylazine solution (Medistar and Serumwerk) (100 mg/kg and 16 mg/kg, respectively) and transcardially perfused with phosphate-buffered saline (PBS), followed by 4% paraformaldehyde (PFA) (1004005, Merck) (w/v) in PBS. Extracted brains were post-fixed overnight at 4 °C in 4% PFA (w/v) in PBS. Brains were either embedded in 6% agarose and sliced using a Vibratome (VT1000S—Leica) or incubated overnight in 25% sucrose, snap frozen in dry ice and sliced using a cryostat (Leica) into 50-μm free-floating coronal sections.
Immunohistochemistry
Brain sections were blocked at room temperature for 2 h in 5% donkey serum (Biozol JIM-017-000-121) diluted in 1X PBS 0.5% TritonX-100 and then incubated with primary antibody at 4 °C overnight in the same solution. Primary antibodies: goat anti-mCherry (1:1000) (Origene AB0040-200), chicken anti-GFP (1:500) (Aves, GFP-1020), rabbit anti-Wfs1 (1:200) (made by the lab of Prof. Dr. Jens F. Rehfeld, University of Copenhagen, Denmark), mouse anti-PKCδ (1:100; 610398, BD Biosciences). After incubation with primary antibody, the sections were washed in 1X PBS (3 × 10 min) and incubated in secondary antibody in 1X PBS 0.5% TritonX-100 at 4 °C overnight. Secondary antibodies: Alexa Fluor donkey anti-rabbit/goat/chicken 488/Cy3/647, (1:300) (Jackson). After 3 × 10 min washes in 1X PBS, sections were incubated in DAPI and coverslipped (Dako).
Microscopy and image processing
A Leica SP8 confocal microscope equipped with a 20×/0.75 IMM or 10×/0.30 FLUOTAR objective (Leica) was used to acquire Fluorescence z-stack images. For projection analysis, images were acquired at the same fixed exposure time and gain using a Leica Thunder microscope and the tile scan and automated mosaic merge functions of Leica LAS X software. Images were minimally processed with ImageJ software (NIH) to adjust for brightness and contrast for optimal representation of the data. For all cell quantifications of brain sections, ImageJ was used to manually count the cells. For the quantification of axon projections we calculated the corrected total fluorescence (CTF) adapted from this protocol (CTCF): https://theolb.readthedocs.io/en/latest/imaging/measuring-cell-fluorescence-using-imagej.html. In brief, on each slide that was used for quantification, we measured the intensity of a square in the ROI (Region of Interest) and the background intensity. The CTF was quantified as: Integrated Density—(Area of selected ROI × Mean fluorescence of background readings). Afterward, the CTFs of the same ROI were averaged and normalized to the injection site. The normalization was performed as: Normalized intensity= IntROI/IntInjectionSite.
Statistical analysis
No statistical methods were used to pre-determine sample sizes. The numbers of samples in each group were based on those in previously published studies. Statistical analyses were performed with Prism 9 (GraphPad) and all statistics are indicated in the figure legends. T-tests or two-way ANOVA with Bonferroni post-hoc tests were used for individual comparisons of normally distributed data. Normality was assessed using Shapiro-Wilk test. If the data were not normally distributed, Mann-Whitney U test and Wilcoxon signed-rank test were performed for individual comparisons. After the conclusion of experiments, virus expression and implants placement were verified. Mice with very low or null virus expression were excluded from analysis.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
Source Data are provided with this paper. Additional raw data from this study are available from the corresponding author upon request. They are not deposited to a public database due to their large size and size limitations of online depositories.
References
Balleine, B. W. & Killcross, S. Parallel incentive processing: an integrated view of amygdala function. Trends Neurosci. 29, 272–279 (2006).
Janak, P. H. & Tye, K. M. From circuits to behaviour in the amygdala. Nature 517, 284–292 (2015).
Davis, M. & Whalen, P. J. The amygdala: vigilance and emotion. Mol. Psychiatry 6, 13–34 (2001).
Everitt, B. J., Cardinal, R. N., Parkinson, J. A. & Robbins, T. W. Appetitive behavior: impact of amygdala-dependent mechanisms of emotional learning. Ann. N. Y. Acad. Sci. 985, 233–250 (2003).
Douglass, A. M. et al. Central amygdala circuits modulate food consumption through a positive-valence mechanism. Nat. Neurosci. 20, 1384–1394 (2017).
Cai, H., Haubensak, W., Anthony, T. E. & Anderson, D. J. Central amygdala PKC-δ(+) neurons mediate the influence of multiple anorexigenic signals. Nat. Neurosci. 17, 1240–1248 (2014).
Kim, J., Zhang, X., Muralidhar, S., LeBlanc, S. A. & Tonegawa, S. Basolateral to central amygdala neural circuits for appetitive behaviors. Neuron 93, 1464–1479.e5 (2017).
Morales, I. & Berridge, K. C. ‘Liking’ and ‘wanting’ in eating and food reward: brain mechanisms and clinical implications. Physiol. Behav. 227, 113152 (2020).
Chen, G. et al. Distinct reward processing by subregions of the nucleus accumbens. Cell Rep. 42, 112069 (2023).
Kargl, D. et al. The amygdala instructs insular feedback for affective learning. eLife 9, e60336 (2020).
Ponserre, M., Fermani, F., Gaitanos, L. & Klein, R. Encoding of environmental cues in central amygdala neurons during foraging. J. Neurosci. 42, 3783–3796 (2022).
Yang, T. et al. Plastic and stimulus-specific coding of salient events in the central amygdala. Nature 616, 510–519 (2023).
Peters, C. et al. Transcriptomics reveals amygdala neuron regulation by fasting and ghrelin thereby promoting feeding. Sci. Adv. 9, eadf6521 (2023).
Livneh, Y. et al. Homeostatic circuits selectively gate food cue responses in insular cortex. Nature 546, 611–616 (2017).
Fadok, J. P., Markovic, M., Tovote, P. & Lüthi, A. New perspectives on central amygdala function. Curr. Opin. Neurobiol. 49, 141–147 (2018).
Han, W. et al. Integrated control of predatory hunting by the central nucleus of the amygdala. Cell 168, 311–324.e18 (2017).
Mike, J. F. R., Shelley, M. W. & Kent, C. B. Optogenetic excitation of central amygdala amplifies and narrows incentive motivation to pursue one reward above another. J. Neurosci. 34, 16567 (2014).
Adke, A. P. et al. Cell-type specificity of neuronal excitability and morphology in the central amygdala. eNeuro 8, ENEURO.0402-20.2020 (2021).
Sevil, D., Daniela, P. & Denis, P. Central amygdala activity during fear conditioning. J. Neurosci. 31, 289 (2011).
LeDoux, J. E., Iwata, J., Cicchetti, P. & Reis, D. J. Different projections of the central amygdaloid nucleus mediate autonomic and behavioral correlates of conditioned fear. J. Neurosci. 8, 2517–2529 (1988).
Ponserre, M., Peters, C., Fermani, F., Conzelmann, K. K. & Klein, R. The insula cortex contacts distinct output streams of the central amygdala. J. Neurosci. 40, 8870–8882 (2020).
Wang, Y. et al. Multimodal mapping of cell types and projections in the central nucleus of the amygdala. eLife 12, e84262 (2023).
Hardaway, J. A. et al. Central amygdala prepronociceptin-expressing neurons mediate palatable food consumption and reward. Neuron 102, 1037–1052.e7 (2019).
Torruella-Suárez, M. L. et al. Manipulations of central amygdala neurotensin neurons alter the consumption of ethanol and sweet fluids in mice. J. Neurosci. 40, 632–647 (2020).
McCullough, K. M., Daskalakis, N. P., Gafford, G., Morrison, F. G. & Ressler, K. J. Cell-type-specific interrogation of CeA Drd2 neurons to identify targets for pharmacological modulation of fear extinction. Transl. Psychiatry 8, 164 (2018).
Dilly, G. A., Kittleman, C. W., Kerr, T. M., Messing, R. O. & Mayfield, R. D. Cell-type specific changes in PKC-delta neurons of the central amygdala during alcohol withdrawal. Transl. Psychiatry 12, 289 (2022).
Yu, K., Garcia da Silva, P., Albeanu, D. F. & Li, B. Central amygdala somatostatin neurons gate passive and active defensive behaviors. J. Neurosci. 36, 6488–6496 (2016).
Wilson, T. D. et al. Dual and opposing functions of the central amygdala in the modulation of pain. Cell Rep. 29, 332–346.e5 (2019).
Fadok, J. P. et al. A competitive inhibitory circuit for selection of active and passive fear responses. Nature 542, 96–100 (2017).
Madisen, L. et al. Transgenic mice for intersectional targeting of neural sensors and effectors with high specificity and performance. Neuron 85, 942–958 (2015).
Fenno, L. E. et al. Targeting cells with single vectors using multiple-feature Boolean logic. Nat. Methods 11, 763–772 (2014).
Fenno, L. E. et al. Comprehensive dual- and triple-feature intersectional single-vector delivery of diverse functional payloads to cells of behaving mammals. Neuron 107, 836–853.e11 (2020).
Becker, J. A. et al. Transcriptome analysis identifies genes with enriched expression in the mouse central extended amygdala. Neuroscience 156, 950–965 (2008).
Luuk, H. et al. Distribution of Wfs1 protein in the central nervous system of the mouse and its relation to clinical symptoms of the Wolfram syndrome. J. Comp. Neurol. 509, 642–660 (2008).
Ray, R. S. et al. Impaired respiratory and body temperature control upon acute serotonergic neuron inhibition. Science 333, 637–642 (2011).
Berndt, A. et al. Structural foundations of optogenetics: determinants of channelrhodopsin ion selectivity. Proc. Natl. Acad. Sci. USA 113, 822–829 (2016).
Schneider, S., Lee, J. H. & Mathis, M. W. Learnable latent embeddings for joint behavioural and neural analysis. Nature 617, 360–368 (2023).
Campos, C. A., Bowen, A. J., Schwartz, M. W. & Palmiter, R. D. Parabrachial CGRP neurons control meal termination. Cell Metab. 23, 811–820 (2016).
Han, S., Soleiman, M. T., Soden, M. E., Zweifel, L. S. & Palmiter, R. D. Elucidating an affective pain circuit that creates a threat memory. Cell 162, 363–374 (2015).
Palmiter, R. D. The parabrachial nucleus: CGRP neurons function as a general alarm. Trends Neurosci. 41, 280–293 (2018).
Campos, C. A., Bowen, A. J., Roman, C. W. & Palmiter, R. D. Encoding of danger by parabrachial CGRP neurons. Nature 555, 617–622 (2018).
Carter, M. E., Soden, M. E., Zweifel, L. S. & Palmiter, R. D. Genetic identification of a neural circuit that suppresses appetite. Nature 503, 111–114 (2013).
Pauli, J. L. et al. Molecular and anatomical characterization of parabrachial neurons and their axonal projections. eLife 11, e81868 (2022).
Michael, C. C. et al. Parabrachial complex: a hub for pain and aversion. J. Neurosci. 39, 8225 (2019).
Biglari, N. et al. Functionally distinct POMC-expressing neuron subpopulations in hypothalamus revealed by intersectional targeting. Nat. Neurosci. 24, 913–929 (2021).
Poulin, J. F. et al. Mapping projections of molecularly defined dopamine neuron subtypes using intersectional genetic approaches. Nat. Neurosci. 21, 1260–1271 (2018).
Britz, O. et al. A genetically defined asymmetry underlies the inhibitory control of flexor–extensor locomotor movements. eLife 4, e04718 (2015).
Sanchez, M. R. et al. Dissecting a disynaptic central amygdala-parasubthalamic nucleus neural circuit that mediates cholecystokinin-induced eating suppression. Mol. Metab. 58, 101443 (2022).
Schnapp, W. I. et al. Development of activity-based anorexia requires PKC-δ neurons in two central extended amygdala nuclei. Cell Rep. 43, 113933 (2024).
Park-York, M., Boghossian, S., Oh, H. & York, D. A. PKCθ expression in the amygdala regulates insulin signaling, food intake and body weight. Obesity 21, 755–764 (2013).
Ip, C. K. et al. Amygdala NPY circuits promote the development of accelerated obesity under chronic stress conditions. Cell Metab. 30, 111–128.e6 (2019).
Steinberg, E. E. et al. Amygdala-midbrain connections modulate appetitive and aversive learning. Neuron 106, 1026–1043.e9 (2020).
Zhao, Z. et al. A novel cortical mechanism for top-down control of water intake. Curr. Biol. 30, 4789–4798.e4 (2020).
Gong, R., Xu, S., Hermundstad, A., Yu, Y. & Sternson, S. M. Hindbrain double-negative feedback mediates palatability-guided food and water consumption. Cell 182, 1589–1605.e22 (2020).
Zhang-Molina, C., Schmit, M. B. & Cai, H. Neural circuit mechanism underlying the feeding controlled by insula-central amygdala pathway. iScience 23, 101033 (2020).
Morton, G. J., Meek, T. H. & Schwartz, M. W. Neurobiology of food intake in health and disease. Nat. Rev. Neurosci. 15, 367–378 (2014).
Chometton, S. et al. A premammillary lateral hypothalamic nuclear complex responds to hedonic but not aversive tastes in the male rat. Brain Struct. Funct. 221, 2183–2208 (2016).
Ch’ng, S., Fu, J., Brown, R. M., McDougall, S. J. & Lawrence, A. J. The intersection of stress and reward: BNST modulation of aversive and appetitive states. Prog. Neuro Psychopharmacol. Biol. Psychiatry 87, 108–125 (2018).
Jennings, J. H., Rizzi, G., Stamatakis, A. M., Ung, R. L. & Stuber, G. D. The inhibitory circuit architecture of the lateral hypothalamus orchestrates feeding. Science 341, 1517–1521 (2013).
Zhang, J. et al. Dissection of the bed nucleus of the stria terminalis neuronal subtypes in feeding regulation. Physiol. Behav. 271, 114333 (2023).
Zhao, Z., Chen, Y., Xuan, Y., Zhou, G. & Qiu, W. Parabrachial nucleus neuron circuits that control feeding behavior and energy balance. Obes. Med. 42, 100509 (2023).
Ryan, P. J., Ross, S. I., Campos, C. A., Derkach, V. A. & Palmiter, R. D. Oxytocin-receptor-expressing neurons in the parabrachial nucleus regulate fluid intake. Nat. Neurosci. 20, 1722–1733 (2017).
Furlan, A. et al. Neurotensin neurons in the extended amygdala control dietary choice and energy homeostasis. Nat. Neurosci. 25, 1470–1480 (2022).
Cui, Y. et al. A central amygdala-substantia innominata neural circuitry encodes aversive reinforcement signals. Cell Rep. 21, 1770–1782 (2017).
Weinberger, N. M. The medial geniculate, not the amygdala, as the root of auditory fear conditioning. Hear Res 274, 61–74 (2011).
Jaramillo, S., Borges, K. & Zador, A. M. Auditory thalamus and auditory cortex are equally modulated by context during flexible categorization of sounds. J. Neurosci. 34, 5291–5301 (2014).
Shi, C. & Davis, M. Visual pathways involved in fear conditioning measured with fear-potentiated startle: behavioral and anatomic studies. J. Neurosci. 21, 9844–9855 (2001).
Salay, L. D. & Huberman, A. D. Divergent outputs of the ventral lateral geniculate nucleus mediate visually evoked defensive behaviors. Cell Rep. 37, 109792 (2021).
Studtmann, C. et al. Ventral posterolateral and ventral posteromedial thalamocortical neurons have distinct physiological properties. J. Neurophysiol. 130, 1492–1507 (2023).
Ding, W., Weltzien, H., Peters, C. & Klein, R. Nausea-induced suppression of feeding is mediated by central amygdala Dlk1-expressing neurons. Cell Rep. 43, 113990 (2024).
Madisen, L. et al. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat. Neurosci. 13, 133–140 (2010).
Lao, Z., Raju, G. P., Bai, C. B. & Joyner, A. L. MASTR: a technique for mosaic mutant analysis with spatial and temporal control of recombination using conditional floxed alleles in mice. Cell Rep. 2, 386–396 (2012).
Chaveroche, M. K., Ghigo, J. M. & d’Enfert, C. A rapid method for efficient gene replacement in the filamentous fungus Aspergillus nidulans. Nucleic Acids Res 28, E97 (2000).
Depaepe, V. et al. Ephrin signalling controls brain size by regulating apoptosis of neural progenitors. Nature 435, 1244–1250 (2005).
Johansson, T. et al. Building a zoo of mice for genetic analyses: a comprehensive protocol for the rapid generation of BAC transgenic mice. Genesis 48, 264–280 (2010).
Raymond, C. S. & Soriano, P. High-efficiency FLP and PhiC31 site-specific recombination in mammalian cells. PLoS ONE 2, e162 (2007).
Acknowledgements
We thank Soo Jin Min-Weissenhorn and the Transgenic Service for help with generating transgenic mouse lines; Jens F. Rehfeld, University of Copenhagen, for providing the Wfs1 antibody; Minh Chau Mai, Emily Russell, Amelie Neubauer for help with management of the animal colonies; Alessandra Monaco for support in cryosectioning; and the company Cergentis B.V. for chromosomal mapping of the Flpo transgenic lines. This study was supported by the Max-Planck Society and the European Research Council under the European Union’s Horizon 2020 research and innovation program (No. 885192; to R.K.).
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
F.F. and R.K. conceptualized the study and designed experiments. F.F. performed most of the experiments. S.C. performed CEBRA and projections intensity analyses. Y.M. assisted with stereotaxic surgeries and the quinine consumption experiments. C.P. performed and analyzed electrophysiology experiments. L.G. assisted with histology, immunohistochemistry, microscopy, and image processing. P.L.A.M. generated the Wfs1-FlpoER transgene, helped designing the PKCδ-Flpo transgene and establishing both transgenic mouse lines. C.R. and K.D. provided intersectional viruses. F.F. and R.K. wrote the manuscript with input from all authors. R.K. supervised and provided funding.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Ivan de Araujo and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Fermani, F., Chang, S., Mastrodicasa, Y. et al. Food and water intake are regulated by distinct central amygdala circuits revealed using intersectional genetics. Nat Commun 16, 3072 (2025). https://doi.org/10.1038/s41467-025-58144-3
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41467-025-58144-3