Abstract
Stem-like CD8⁺ exhausted T cells (Tex) sustain antitumor immunity, whereas TGFβ signaling acts as a major immunosuppressive pathway. In patients with colorectal liver metastases, we observe that elevated TβRI expression in peri-metastatic hepatocytes correlates with poor prognosis. We therefore investigate whether disrupting hepatocytic TGFβ signaling can reinvigorate stem-like CD8⁺ Tex cells to restrict liver metastasis. In support of this hypothesis, mice with hepatocyte-specific TβRI depletion exhibit reduced liver metastatic burden across multiple tumor models. Mechanistically, hepatocytic TβRI blockade suppresses Galectin-9 secretion, which reshapes the transcriptional program of intra-tumoral CD8⁺ T cells. This reprogramming promotes a phenotypic transition from terminal exhaustion toward stem-like and effector states, yielding T cell subsets with enhanced metastasis-control capacity. Importantly, this axis functions independently of macrophages and CD4⁺ T cells. Furthermore, therapeutic delivery of Galunisertib using choline-modified lipid nanoparticles synergizes with αPD-1, fostering the conversion of exhausted CD8⁺ T cells into responsive Ly108⁺CX3CR1⁺ subsets and suppressing liver metastases. Collectively, our results identify hepatocyte TGFβ signaling as a targetable checkpoint against liver metastases.
Similar content being viewed by others
Introduction
Metastasis is notorious for its life-limiting impact on cancer patients, with the liver emerging as the predominant site for distant metastasis across various primary cancers1. The hepatic immunotolerant environment is currently acknowledged as the primary contributor to liver organotropism2. Despite the revolutionary advancements in immunotherapy, particularly immune checkpoint blockade (ICB), individuals with liver metastasis derive less benefit3. The co-option of disseminated tumor cells with surrounding immune cells and organ-specific non-immune stromal cells facilitates their seeding and colonization4. Consequently, there is an urgent imperative to investigate the mechanisms through which cancer cells orchestrate the co-evolution of local cells, leading to tumor progression and intrinsic resistance to ICB5.
Exhausted T cells (Tex) are defined as a specific lineage exhibiting progressive and hierarchical loss of effector function6,7. Within this population, distinct subsets of Tex exhibit varying degrees of functionality and differentiation. PD1+ TCF1+Ly108+ stem-like Tex maintain self-renewal potential and are critical for sustaining durable CD8+ T cell responses over time, serving as a reservoir for replenishing more differentiated effector cells8. Conversely, PD1+TCF1-Ly108- terminally differentiated Tex are severe dysfunction and short-lived in the tumor microenvironment9.
The persistence of stem-like Tex is pivotal for effective immunotherapy, as these cells underpin long-term T cell responses and tumor control10. However, a disproportionate expansion of terminally differentiated Tex in the tumor microenvironment contributes to immune suppression and limits the efficacy of ICB11. Understanding the hierarchical differentiation trajectory of Tex, particularly within metastatic niches, is essential to overcoming their hypo-responsiveness and developing strategies to reinvigorate Tex subsets for improved therapeutic outcomes.
TGFβ serves as the master regulator in shaping the immunosuppressive microenvironment of tumors12. TGFβ activation is considered a core marker for subtyping mesenchymal colorectal cancer (CRC)13. Our previous studies have demonstrated that TGFβ-driven epithelial-mesenchymal transition is indispensable for promoting liver metastasis14,15. Recent reports provide further evidence supporting the notion that tumor-derived TGFβ facilitates liver metastasis by establishing an immunologically permissive niche16. In chronic infections and cancer, TGFβ promotes the expression of inhibitory receptors, thereby imposing an exhausted phenotype on CD8+ T cells, leading to a state of generalized immunosuppression17. It is intriguing to exploring how hepatic TGFβ signaling determines liver metastasis by finely tuning Tex-mediated immune responses.
Building on these insights, we hypothesize that hepatocyte-intrinsic TGFβ signaling critically shapes the metastatic niche by modulating CD8⁺ T cell exhaustion. Using transgenic models, we demonstrate that TGFβ-responsive hepatocytes drive metastasis progression by suppressing stem-like T cell populations, and that targeting this pathway not only reduces metastatic burden but also synergizes with αPD-1 therapy. These findings reveal a previously underappreciated role for hepatocytes in orchestrating immune evasion, suggesting that stromal reprogramming may represent a viable strategy to overcome immunotherapy resistance in patients with liver metastases.
Results
TβRI-hKO restricts liver metastases across multiple tumor types
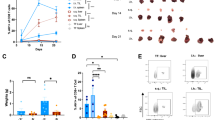
To investigate the spatial regulation of hepatic TGFβ signaling in liver metastasis, we performed immunohistochemical profiling on 36 microsatellite-stable (MSS), moderately differentiated CRLM specimens from patients without any neoadjuvant therapy (Supplementary Table 1). Elevated TβRI expression was localized to peri-metastatic niches in 75% of cases (27/36), forming receptor-enriched stromal compartments distinct from distant hepatic parenchyma and metastatic lesions (Fig. 1a and Supplementary Fig. 1a). Critically, patients with TβRI-high peri-metastatic niches exhibited significantly shortened median overall survival, establishing hepatic TGFβ signaling as a prognostic biomarker in CRLM (Fig. 1b and Supplementary Table 1). Multiplex immunohistochemistry (m-IHC) resolved cellular specificity within these niches: TβRI expression was predominantly localized to hepatocytes in peri-metastatic niches, with scattered detection in hepatic stellate cells (HSCs), while showing minimal association with tumor-associated macrophages (TAMs) (Fig. 1c). Strikingly, 70% of TβRI-high cases demonstrated concurrent phosphorylated Smad2 (p-Smad2) in peri-metastatic hepatocytes, indicative of active receptor-driven signaling (Supplementary Fig. 1b). In contrast, metastatic tumor cells exhibited ligand-dependent p-Smad2 activation despite low TβRI expression, revealing a compartmentalized signaling paradigm: hepatic receptor activation at the tumor interface versus tumor-intrinsic ligand dependency within lesions (Supplementary Fig. 1b–f). While TGFβ-mediated HSCs activation is known to drive metastasis via extracellular matrix remodeling and immunosuppression18, the role of hepatocyte-specific TGFβ signaling remains poorly defined. To address this, we generated hepatocyte-specific TβRI knockout mice (TβRI-hKO) and established liver metastasis models using immunotherapy-resistant (B16F10 melanoma, PAN02 pancreatic cancer) and -sensitive (MC38 colorectal cancer) cell lines (Fig. 1d and Supplementary Fig. 1g–i). Notably, metastatic colonization at Day 7 was comparable between TβRI-hKO and wild-type (TβRI-hWT) mice, indicating that loss of hepatocytic TGFβ signaling does not impair initial tumor engraftment. However, TβRI-hKO mice consistently exhibited reduced metastatic burden at later stages across all models, demonstrating that hepatocyte-derived TGFβ signaling is a non-redundant driver of metastatic progression, independent of tumor immunogenicity or HSC activity (Fig. 1e–l). Comprehensive immune profiling revealed preserved total numbers of CD4+/CD8+ T cells, myeloid-derived suppressor cells (PMN-/Mo-MDSCs), dendritic cells, and NK/NKT cells in TβRI-hKO versus TβRI-hWT mice. Strikingly, TAMs were significantly reduced in TβRI-hKO mice (Supplementary Fig. 2a, b).
a IHC staining with TβRI-specific antibody to detect the distribution of TβRI+ cells in colorectal liver metastases (n = 36 patients). Representative IHC image. Scale bar, 50 μm. The bar chart in the right represents the percentage of high and low TβRI expression cases in liver metastasis, adjacent and distal liver tissues. p < 0.001 by two-sided Pearson’s chi-square test. b Overall survival curves based on adjacent liver TβRI expressed level in CRLM patients using the multivariate COX regression analysis, a two-sided P-value of < 0.05 was considered statistically significant. c Representative images depicting multicolor immunohistochemical co-staining of TβRI with specific cell type markers in CRLM adjacent liver tissues (DAPI: cell nucleus, HNF4α: hepatocytes, α-SMA: hepatic stellate cells, CD68: macrophages, PAN-CK: tumor cells). Scale bar, 25 μm. d Experimental scheme for generating TβRIflox/flox Alb-Cre+/- (TβRI-hKO) mice. TβRIflox/flox Alb-Cre-/- and C57BL/6 J were used as TβRI-hWT mice. e Representative bioluminescent image and Quantification of TβRI-hWT versus TβRI-hKO mice inoculated with MC38-Luc cells (n = 6 mice/group). f Representative macroscopic image of (e) and Quantification of Liver to body weight ratio (LBR). g Representative bioluminescent image of TβRI-hWT versus TβRI-hKO mice inoculated with PAN02-Luc cells (n = 5 mice/group). h Quantification of (g). i Representative macroscopic image of (g) and Quantification of LBR. j Representative bioluminescent image of TβRI-hWT versus TβRI-hKO mice inoculated with B16F10-Luc cells (n = 5 mice/group). k Quantification of (j). l Representative macroscopic image of (j) and Quantification of LBR. Data are presented as the mean ± SD, and were analyzed with an unpaired two-sided Student’s t test (e–l). Consistent results were observed across three biological replicates in (e–l). Source data are provided as a Source Data file.
Given the centrality of CD8+ T cell functionality—rather than abundance—in antitumor immunity, we hypothesized that hepatocyte TGFβ signaling regulates metastatic progression by steering CD8+ T cell differentiation trajectories. To test this, we mapped the fate of tumor-reactive CD8+CD44+ T cells from memory precursor effector cells (MPECs) to terminal exhaustion states (Supplementary Fig. 2c). While TβRI-hKO modestly enriched a KLRG1+PD1− effector-like subset, the majority of CD8+ T cells in advanced metastases (Days 21–28) adopted a KLRG1−PD1+ exhausted phenotype (Tex) across all models (Supplementary Fig. 2d–g). Strikingly, TβRI-hKO suppressed terminal Tex differentiation, marked by co-expression of PD1hi, TIM3, TIGIT and LAG3. However, a mere 10% increase in PD1int progenitor cells was observed on day 21 after MC38 inoculation, absent on day 14 and day 28 following B16F10 and PNA02 injection (Supplementary Fig. 2h–j). This divergence highlights that hepatocyte TGFβ signaling primarily accelerates terminal exhaustion rather than broadly suppressing progenitor maintenance. Collectively, these findings establish hepatocyte TGFβ signaling as an orchestrator of CD8+ T cell terminal exhaustion, fostering an immunosuppressive niche that enables liver metastasis progression.
TβRI-hKO enhances the differentiation of intratumor Ly108+CX3CR1- stem-like and Ly108+CX3CR1+ stem-like effector CD8+ Tex subsets
Given that CX3CR1+ CD8+ T cells display enhanced cytotoxicity and cell-cycle activity in chronic viral infection19,20, we hypothesized that TβRI-hKO might ameliorate liver metastasis by triggering the differentiation of CX3CR1+ phenotype. To this end, we analyzed the frequency of functional T cell subsets identified by the expression of CX3CR1 and Ly108, with the latter commonly used as a surrogate marker for TCF18 (Supplementary Fig. 3a). Contrary to our expectations, TβRI-hKO resulted in a significant contraction of Ly108-CX3CR1+ subset in the metastatic microenvironment, which is completely different from the phenotype in chronic virus infection. Interestingly, the frequency of both Ly108+CX3CR1- and Ly108+CX3CR1+ subsets markedly increased in TβRI-hKO mice on day 14, day 21 and day 28 after B16F10, MC38 or PAN02 inoculation, respectively. Correspondingly, we noticed an expected reduction of Ly108-CX3CR1- terminal differentiated Tex under the background of TβRI-hKO. We then conducted a comprehensive investigation into the anatomical distribution of the four subsets and found that TβRI-hKO significantly augmented the frequency of both Ly108+CX3CR1- and Ly108+CX3CR1+ subsets in the tumor stroma, but not in the blood or spleen (Fig. 2a, b).
a, b Frequency of T cell subsets based on the expression of Ly108 and CX3CR1 within CD8+ PD1+CD44+ tumor-reactive T cells in different anatomical locations of TβRI-hWT and TβRI-hKO mice (n = 6 mice/group). Flow cytometry representative plots (a). Statistical analysis of these subsets in liver metastases across multiple tumor types are shown (b). Statistical analysis of these subsets in the spleen and PBMC are not shown. c–f Flow cytometry was used to detect the phenotypes, transcriptional profiles, and effector functions of Ly108+CX3CR1-, Ly108+CX3CR1+, Ly108-CX3CR1+, Ly108-CX3CR1- subsets in liver metastases of TβRI-hWT and TβRI-hKO mice inoculated with PAN02 on day 28. c Representative histograms showing the expression of PD-1, LAG3, TIGIT, TIM3, and 2B4 among these T subsets (n = 6 mice/group). MFI analysis of TIM3 and 2B4 was summarized in the right statistical chart. d Representative flow cytometry plots and statistical analysis depicting the TOX/TCF1 co-expression among these T subsets (n = 4 mice/group). e Representative flow cytometry plots and statistical analysis depicting the TNF and IFNγ co-production by these T subsets upon ex vivo CD3/CD28 stimulation (n = 5 mice/group). f Representative flow cytometry plots and statistical analysis depicting the percentages of these T subsets degranulating (CD107a) and producing IL2 upon ex vivo CD3/CD28 stimulation (n = 5 mice/group). Data are presented as the mean ± SD. Data were analyzed with unpaired two-sided Student’s t test (b) and two-way ANOVA with Tukey’s multiple comparisons test (c–f). Consistent results were observed across three biological replicates in (a–f). Source data are provided as a Source Data file.
As the redistribution of the four subsets correlated with tumor control, we aimed to elucidate the effector versus exhaustion characteristics of these subsets. By monitoring the five indicated IRs, we discovered that the two Ly108- subsets expressed higher levels of both TIM3 and 2B4 as compared to Ly108+CX3CR1- and Ly108+CX3CR1+ subsets. The upregulation of these two IRs indicated a terminally exhausted phenotype in the Ly108-negative subsets21. Meanwhile, TβRI-hKO markedly reduced the expressions of both TIM3 and 2B4 among these four subsets (Fig. 2c). Nevertheless, the expressions of PD1, LAG3 and TIGIT remained consistently high in the other three Tex subsets, except in the Ly108+CX3CR1- subset (Supplementary Fig. 3b). This suggests considerable heterogeneity in their development towards the exhausted phenotype.
We further visualized that the clustering of the Ly108-CX3CR1- subset was accompanied by a sharp decline of TCF1 and a notable increment of TOX and Eomes (Supplementary Fig. 3c). Meanwhile, TβRI-hKO induced robust TCF1 expression but weakened TOX expression among both Ly108+CX3CR1- and Ly108+CX3CR1+cells compared to the corresponding subsets in TβRI-hWT mice (Fig. 2d). Additional evidence proved that the capacity of CD8+ T cells to acquire a less exhausted phenotype in TβRI-hKO relied heavily on TCF1, but not on T-bet (Supplementary Fig. 3d). Interestingly, we noticed that the Ly108-CX3CR1- subset showed the highest proliferation rate in vivo, whereas Ly108+CX3CR1- subset exhibited the lowest proliferation capacity which was consistent with the fact that TCF1 promoted the stemness of CD8+ T cells (Supplementary Fig. 3e). TβRI-hKO obviously enhanced the stemness, increased the number of the two Ly108+ fractions and limited the exhaustion of the two Ly108- fractions.
We next evaluated effector functions upon anti-CD3/CD28 stimulation ex vivo. Among the Tex subsets, Ly108+CX3CR1+ cells exhibited the strongest capacity to produce pro-inflammatory cytokines. The number of IFNγ-producers and IFNγ & TNF co-producers in Ly108+CX3CR1- cells was close to that in Ly108+CX3CR1+ cells, but to a lesser extent. Consistent with their terminally exhausted phenotype, Ly108-CX3CR1+ cells and Ly108-CX3CR1- cells had limited ability to produce IFNγ and TNF (Fig. 2e). In addition, two Ly108+ fractions displayed an enhanced capacity to produce IL2, indicating an important role for stem-like properties in maintaining CD8+ T cell function (Fig. 2f). We also observed that Ly108-CX3CR1+cells contained the highest frequency of CD107a positive cells (Supplementary Fig. 3f). However, analysis of IL2-producing & CD107a-positive cells showed that Ly108+CX3CR1+ subset had the highest frequency of IL2+CD107a+ cells (Fig. 2f), further reinforcing the effector traits of this Tex subset in our liver metastatic model. Taken together, hepatocytic TβRI ablation resulted in the aggregation of Ly108+CX3CR1- and Ly108+CX3CR1+ subsets, leading to an increase in their cytokine production (IL2, TNF, IFNγ).
Ly108+CX3CR1- and Ly108+CX3CR1+ subsets demonstrate superior metastasis-control efficacy upon adoptive transfer
To investigate the efficacy of these Tex subsets in the control of liver metastases, we performed adoptive transfer experiments using congenically marked CD8+ T cells. To do this, Ly108+CX3CR1- and Ly108+CX3CR1+ cells were purified from TβRI-hKO mice, whereas Ly108-CX3CR1- and Ly108-CX3CR1+ cells were purified from TβRI-hWT mice at D21 post-inoculation of PAN02 cells into the portal circulation. Subsequently, we separately transferred these subsets intravenously into tumor-matched C57B6L/J mice (Fig. 3a). Bioluminescence showed that both Ly108+ subsets exhibited the most pronounced capacity to restrict liver metastasis among these four subsets, while their counterparts in the other two Ly108- subsets had limited contribution to controlling tumor growth (Fig. 3b–d).
a Experimental design. b Representative bioluminescent image of pre-adoptive and post-adoptive C57BL/6 J mice receiving Ly108+CX3CR1-, Ly108+CX3CR1+, Ly108-CX3CR1+, Ly108-CX3CR1- cells transfer or without transfer (n = 5 mice/group). c Quantification of (b). d Survival curve of tumor-bearing mice for indicated groups described in (b). Log-rank test for survival comparison. e Flow cytometry depicted the phenotype of adoptively transferred cells before and 14 days after transfer. All subsets shown were gated on CD8+PD1+CD44+CD45.2+CD45.1- cells. f Representative bioluminescent image of TβRI-hWT and TβRI-hKO mice inoculated with TGFβ1OE, TGFβ1KD, or TGFβ1CTL PAN02-Luc cells (n = 6 mice/group). g Quantification of (f). Data are presented as the mean ± SD, and were analyzed with one-way (g) or two-way (c) ANOVA with Tukey’s multiple comparison test. Consistent results were observed across three biological replicates in (b–g). Source data are provided as a Source Data file.
We further evaluated the in vivo differentiation trajectory of these four subsets during liver metastatic progression. Two weeks post-transfer, CD8+ T cells were isolated from liver metastasis of recipient mice and analyzed by flow cytometry for the expression of Ly108 and CX3CR1 (Fig. 3a). Our findings indicated that Ly108+CX3CR1- cells could regenerate all four Tex populations, whereas Ly108+CX3CR1+ cells only gave rise to Ly108-CX3CR1+ cells. Remarkably, Ly108-CX3CR1+ cells had limited capacity to differentiate into Ly108-CX3CR1- cells, and both populations predominantly retained their original state (Fig. 3e). These results unveil novel divergent Tex differentiation trajectories during liver metastasis, highlighting the indispensable roles of stem-like properties for tumor control by CD8+ T cells22.
Next, we engineered PAN02-LUC cell lines with overexpression (TGFβ1OE), knockdown (TGFβ1KD), and control (TGFβ1CTL) constructs to underscore the pivotal role of TGFβ1 in liver metastasis (Supplementary Fig. 4a, b). The TGFβ1OE construct was designed to induce host cells to secrete active TGFβ123 (Supplementary Fig. 4c). Mice lacking TβRI and those with intact TβRI were challenged with these constructs for three weeks. Immunohistochemical analysis revealed that TGFβ1 overexpression significantly upregulated the expression of p-Smad2 in hepatocytes compared to TGFβ1 control in TβRI-hWT mice, while knockdown of TGFβ1 reduced the expression of p-Smad2 in hepatocytes (Supplementary Fig. 4d). Bioluminescent results revealed that TGFβ1OE significantly accelerated malignant progression, whilec TGFβ1KD moderately restrained tumor growth in TβRI-competent mice. TβRI-hKO, as anticipated, substantially reduced the liver metastatic burden induced by each construct compared to their corresponding genotypes, respectively (Fig. 3f, g). Correspondently, we found that TGFβ1OE led to a majestic enhancement of Ly108-CX3CR1- cells at the expense of the other three subsets in TβRI-hWT mice. Conversely, TGFβ1KD resulted in higher percentages of the two Ly108+ subsets accompanied by a modest restriction on the two Ly108- subsets. Strikingly, robust assembly of Ly108+CX3CR1- subset and Ly108+CX3CR1+ subset was observed in TβRI-absent mice, irrespective of the intensity of TGFβ1 signaling. TβRI-hKO drastically rescued the tumor-driven dysfunctional state of T cells in both TGFβ1OE, TGFβ1KD, or TGFβ1CTL metastatic models (Supplementary Fig. 4e). In hindsight, regardless of the TGFβ1 level, TβRI-hKO consistently provided metastasis-protective benefits by redirecting CD8 responding T cells to a stem-like assembly.
Functional maintenance of stem-like traits in CD8+ T cells by TβRI-hKO is TAMs-independent
Our observation of a more pronounced decline in TAMs in TβRI-hKO mice compared to TβRI-hWT mice promoted us to examine the role of these cells in the heightened T cell immunity resulting from hepatocytic-TβRI ablation24. Using CLD liposomes to deplete phagocytic myeloid cells in both TβRI-hWT and TβRI-hKO mice25, we initiated treatment every three days for 15 days, starting from the fourth day after PAN02 cell inoculation (Fig. 4a). Our data indicate that in TβRI-hWT mice, the M1/M2 ratio decreases on day 21 post-CLD treatment, suggesting that pro-inflammatory M1 macrophages are predominantly cleared at this early stage. By day 28, there is a shift toward an increased population of immunosuppressive M2 macrophages, and the M1/M2 ratio in the vehicle group approaches unity. After CLD treatment, the ratio stabilizes, indicating a depletion of both M1 and M2 populations over time. In contrast, TβRI-hKO mice consistently exhibit a lower M1/M2 ratio following CLD treatment, maintaining a stable predominance of M1 macrophages (Fig. 4b and Supplementary Fig. 5a–c). In addition, the clearance of TAMs from the liver by CLD in both TβRI-hWT and TβRI-hKO mice proceeded as expected, with no significant or persistent alterations in MDSC subsets observed (Supplementary Fig. 5d). Notably, CLD-mediated macrophage depletion significantly reduced the liver metastatic burden in TβRI-hWT mice compared to vehicle-treated controls. However, in TβRI-hKO mice, TAMs depletion DID not reverse the observed inhibition of liver metastasis relative to vehicle-treated TβRI-hKO counterparts. (Fig. 4c–e).
a Experimental setup to assess the indispensable role of TAMs or CD8+ T cells in improving metastatic outcomes of PAN02-bearing mice resulting from hepatocytic-TβRI ablation. b The M1-to-M2 ratio. c Representative bioluminescent image of TβRI-hWT and TβRI-hKO mice treated with vehicle (veh), Clodronate (CLD) liposome or αCD8 (n = 5 mice/group). d Survival curve of tumor-bearing mice for the indicated groups described in (c). Log-rank test for survival comparison. e Quantification of (c). f Representative flow cytometry plots and Statistical analysis depicting the percentages of Tex subsets in liver metastases of veh or CLD liposome-treated TβRI-hWT and TβRI-hKO mice on days 28. g Representative bioluminescent image and liver macroscopic image of TβRI-hWT and TβRI-hKO mice inoculated with PAN02 + BMDM or PAN02 alone (n = 5 mice/group). h Quantification of (g). i Statistical analysis depicting the percentages of Tex subsets about the indicated groups. Data are presented as the mean ± SD, and were analyzed with one-way (h) or two-way (b, e, f, i) ANOVA with Tukey’s multiple comparison test. Consistent results were observed across three biological replicates in (b–i). Source data are provided as a Source Data file.
Flow cytometry analysis revealed that Macrophage depletion with CLD liposomes had no effect on clustering of two stem-like CD8+ T cells, and decreasing of Ly108-CX3CR1- terminal CD8+ T cells caused by hepatocytic-TβRI ablation (Fig. 4f and Supplementary Fig. 5f). To verify the essential role of CD8+ T cells in liver metastasis suppression by hepatocytic TβRI ablation, we applied an anti-CD8 antibody to neutralize CD8+ T cells in vivo. In TβRI-hKO mice, CD8+ T cell neutralization led to a notable resurgence of liver metastasis compared to vehicle-treated mice (Fig. 4c–e).
To further assess the role of TAMs in the induction of stem-like Tex aggregation by hepatocyte TβRI depletion, we sorted bone marrow-derived macrophages (BMDM) from CD45.1 mice and co-injected them in a 1:1 ratio with PAN02 cells into CD45.2 recipient mice (Supplementary Fig. 5e). Bioluminescence imaging revealed that BMDM supplementation significantly increased the liver metastatic burden in TβRI-hWT mice. Conversely, BMDM supplementation did not reverse the limited liver metastasis phenotype in TβRI-hKO mice (Fig. 4g, h) nor influence the accumulation of stem-like CD8+ T cells associated with hepatocytic TβRI deficiency (Fig. 4i). Overall, these findings confirm that hepatocyte-specific TβRI deficiency enhances the CD8+ T cell-mediated anti-tumor response independently of macrophage modulation.
Loss of Galectin-9 in hepatocytes reverses the pro-metastatic phenotype of TGFβ1-overexpression in tumor cells
Our next endeavor was to elucidate the regulatory mechanisms governing the lineage differentiation of CD8+ T cells under the influence of hepatocytic TGFβ signaling. Initially, we assessed inflammatory cytokines and chemokines in the tumor interstitial fluid using a Luminex multiplex assay. In comparison with TβRI-hWT mice, TβRI-hKO mice exhibited increased expression of IL1β, CCL3, CCL12, and Galectin-3, while a significant reduction of Galectin-9 was observed (Fig. 5a). Given that TGFβ augmented the expression of TIM3 on Ly108-CX3CR1- cells and imposed a terminal exhausted phenotype in this subset, we explored the potential involvement of Galectin-9, a ligand for TIM3, in lineage differentiation26,27. We observed an elevated Galectin-9 secretion in tumor interstitial fluid concurrent with an escalation of TGFβ1 in tumor stroma (Supplementary Fig. 6a). Correlation analysis revealed a consistent relationship between the production of Galectin-9 and TGFβ1 in the context of tumor inoculation (Supplementary Fig. 6b). The expression of Galectin-9 was directly induced by TGFβ1 in hepatocyte cell line AML12 (Supplementary Fig. 6c). Of note, the TGFβ1-driven upregulation of Galectin-9 in tumor interstitial fluid was completely counteracted in hepatocytic TβRI-absent mice. Immunohistochemical analysis revealed that TGFβ1 overexpression significantly upregulated Galectin-9 expression in hepatocytes adjacent to liver metastasis compared to the TGFβ1 control in TβRI-hWT mice. Conversely, knockdown of TGFβ1 led to a reduction in Galectin-9 expression in adjacent hepatocytes. In contrast, Galectin-9 expression was nearly abolished in TβRI-hKO mice compared to TβRI-hWT mice, and Galectin-9 levels were significantly diminished in hepatocytes located in distal regions of liver metastases, as confirmed by analysis of both human and mouse tissue samples (Supplementary Fig. 6d, e). These findings underscore the candidacy of Galectin-9 as a liaison molecule for hepatocytic TGFβ signaling in driving terminal Tex differentiation28.
a Luminex showing the protein levels of inflammatory cytokines in tumor interstitial fluid of TβRI-hWT and TβRI-hKO mice (n = 3 mice/group). Data are shown as a heatmap. b Experimental scheme for generating Gal-9flox/flox Alb-Cre+/- (Gal9-hKO) mice. Gal-9flox/flox Alb-Cre-/- and C57BL/6 J were used as Gal9-hWT mice. c Representative bioluminescent image of Gal9-hWT versus Gal9-hKO mice inoculated with TGFβ1OE or TGFβ1CTL-PAN02 cells in the portal vein on day 28 (n = 6 mice/group). d Bioluminescence quantification of (c). e Statistical analysis depicting the percentages of CD39+TOX+ cells within CD8+PD1+CD44+ tumor-reactive T cells in the indicated groups. f Statistical analysis depicting the percentages of Tex subsets based on TCF1 and CX3CR1 within CD8+PD1+CD44+ tumor-reactive T cells. g statistical analysis depicting the TNF and IFNγ co-production by CD8+ CD44+ T subsets in the indicated groups upon ex vivo CD3/CD28 stimulation. h The diagram of experimental design. i Representative bioluminescent image of Gal9-hWT versus Gal9-hKO mice treated with IgG, αCD4, αCD8 or αPD-1(n = 5 mice/group). j Representative macroscopic image of (i). k Bioluminescence quantification of (i). l Statistical analysis depicting the percentages of Tex subsets based on Ly108 and CX3CR1 within CD8+PD1+CD44+ tumor-reactive T cells. Data are presented as the mean ± SD, and were analyzed with unpaired two-sided Student’s t test (k) or two-way (d, e, f, g, l) ANOVA with Tukey’s multiple comparison test. Consistent results were observed across three biological replicates in (a, c–g, i–l). Source data are provided as a Source Data file.
To confirm Galectin-9 is a key downstream molecule of hepatocytic TGFβ signaling, we generated mice with a hepatocyte-specific knockout of Galectin-9 (Gal9-hKO) by crossing Gal9flox/flox mice with Albumin-Cre mice (Fig. 5b and Supplementary Fig. 6f, g). Bioluminescence showed that the loss of Galectin-9 in hepatocytes significantly rescued the malignant progression of liver metastases caused by TGFβ1 overexpression (Fig. 5c, d). Massive accumulation of TOX in Tex was observed in the context of TGFβ1 overexpression in Gal9-hWT mice, however, Gal9-hKO markedly prevented this accumulation to obstacle terminal Tex differentiation (Fig. 5e). Furthermore, TCF1+CX3CR1- stem-like and TCF1+CX3CR1+ stem-like effector cells were significantly increased in Gal9-hKO mice even when TGFβ signaling was excessive activation. In contrast, TCF1-CX3CR1+ and TCF1-CX3CR1- cells were rare in Gal9-hKO mice but abundant in Gal9-hWT mice (Fig. 5f and Supplementary Fig. 7a). Concurrently, cytokine co-producers markedly accumulated in responding CD8+ T cells in Gal9-hKO mice (Fig. 5g). Taken together, these results illustrate that loss of Galectin-9 in hepatocytes promotes CD8+ T cell-mediated anti-metastasis activity to rescue the malignant progression of liver metastases caused by excessive TGFβ signaling activation.
Given the pivotal role of Galectin-9 in regulating CD4+ T cell responses27,29, we further examined the differentiation pattern of CD4+ T cells (Supplementary Fig. 7b). Compared with Gal9-hWT mice, Gal9-hKO mice exhibited a slight increase in the proportion of Th1 cells (Supplementary Fig. 8a) and a slight decrease in the proportion of Th2/Treg cells (Supplementary Fig. 8b, d), with no significant change in the infiltration of Th17 cells (Supplementary Fig. 8c). To further explore the involvement of CD4+ T cells in the Gal9-hKO-mediated inhibition of liver metastasis, we used neutralizing antibodies to deplete CD4+ T cells in vivo (Fig. 5h). Interestingly, CD4+ T cell depletion DID not impact the inhibitory effect on liver metastasis induced by Gal9-hKO, suggesting that hepatocyte-derived Gal9 regulation of liver metastasis operates independently of CD4+ T cells (Fig. 5i–k).
In contrast, CD8+ T cell depletion in vivo completely reversed the metastasis inhibition induced by Gal9 deficiency in hepatocytes, confirming that this effect is CD8+ T cell-dependent. Furthermore, administration of αPD1 to Gal9-hKO mice produced a synergistic reduction in liver metastasis burden (Fig. 5i–k). This combination treatment also significantly increased the proportion of LY108+CX3CR1+ stem-like and effector CD8+ T cells (Fig. 5l). These findings highlight the potential of targeting hepatocyte Gal9 in conjunction with αPD1 therapy as an effective strategy to inhibit liver metastasis.
Monotherapy with αGal-9 or Galunisertib attenuated liver metastases without synergistic effects
The above findings prompted us to explore whether therapeutic interventions, specifically Galectin-9 neutralization (αGal-9) or TβRI inhibition (Galunisertib)30, would similarly alleviate liver metastasis by reshaping CD8+ T cell responses in our mouse model. Following portal vein transplantation of B16F10 into immune-competent C57BL/6 J mice, the animals were assigned to four groups (Vehicle + IgG, Galunisertib + IgG, Vehicle + αGal-9, Galunisertib + αGal-9) for a 3-week treatment (Fig. 6a). αGal-9 monotherapy significantly reduced the total photon flux in the liver compared to the isotype control. As expected, Galunisertib further delayed tumor growth compared to either the isotype control or αGal-9 monotherapy (Fig. 6b, c). However, the combination therapy of αGal-9 and Galunisertib conferred no additional tumor protection (Fig. 6d), suggesting a mechanistic redundancy between the two intervention approaches. Taken together, both αGal-9 neutralization and TβRI inhibition alone were sufficient to induce anti-tumor immunity.
a Experimental setup to assess the effects of αGal-9 or Galunisertib monotherapy and combination therapy in improving outcomes in B16F10-bearing C57BL/6 J mice with liver metastases. (n = 6 mice, Vehicle + IgG; n = 6 mice, Galunisertib + IgG; n = 6 mice, Vehicle + αGal-9, n = 5 mice, Galunisertib + αGal-9). b Representative bioluminescent and macroscopic image of B16F10-bearing C57BL/6 J mice for indicated groups describe in (a). c Quantification of bioluminescence and LBR. d Survival curve of tumor-bearing mice for the indicated groups described in (b). Log-rank test for survival comparison. e Representative flow cytometry plots depicting the percentages of Tex subsets based on TCF1and CX3CR1. f Statistical analysis of (e). g Representative flow cytometry plots depicting the percentages of Tex subsets based on TOX/CX3CR1. h Statistical analysis of (g). i Representative flow cytometry plots based depicting the percentages of Tex subsets on Ki-67 and TCF1. j Statistical analysis of (i). k–m Representative flow cytometry plots (k) and statistical analysis (l, m) depicting the TNF and IFNγ co-production by CD8+ T cells upon ex vivo CD3/CD28 stimulation for the indicated groups. Data are presented as the mean ± SD, and were analyzed with one-way (c, l, m) or two-way (f, h, g) ANOVA with Sidak’s multiple comparison test. Consistent results were observed across three biological replicates in (b–m). Source data are provided as a Source Data file.
To assess the protective capacity of CD8+ responding T cells in the therapeutic context, we isolated and analyzed intra-tumoral CD8+ T cells from freshly resected tumor samples. Galunisertib led to a 4-fold increase in CX3CR1+TCF1+TOX- stem-like effector CD8+ T cells, accompanied by a substantial reduction in the terminally differentiated CX3CR1-TCF1-TOX+ subset in the tumor nest compared to control (Fig. 6e–h). Treatment with Galunisertib, αGal-9, or the combination therapy significantly augmented the stemness of tumor-reactive CD8+ T cells, as indicated by the enhanced frequency of TCF1+Ki67- populations. The proportion of TCF1-Ki67+ cells correspondingly decreased in the groups treated with Galunisertib, αGal-9, or the combination, signifying a shift from a terminally exhausted phenotype to a less differentiated, stem-like phenotype (Fig. 6i, j). Thus, Galectin-9 neutralization or TβRI inhibition elicited a reservoir of CX3CR1+ TCF1+ stem-like effector CD8+ T cells in the tumor nest to sustain the ongoing antitumor immune response.
Functionally, although the amount of IFNγ-producing tumor-reactive CD8+ T cells showed no noticeable alteration in Galunisertib-treated mice after anti-CD3/CD28 stimulations ex vivo, the T cells from these mice exhibited an enhanced functional capacity, as indicated by a 2-fold increase in the proportion of TNF +IFNγ+ cells after restimulation (Fig. 6k–m). Consistent with these findings, the combination therapy showed nearly the same pattern in the functions of tumor-reactive CD8+ T cells as Galunisertib monotherapy, including stem-like phenotype conversion and improved effector functions. As expected, αGal-9 monotherapy mirrored the effects of Galunisertib monotherapy in reshaping the landscape of tumor-reactive CD8+ T cells, but to a lesser extent. This provided additional evidence supporting the notion that Galectin-9 is part of TGFβ signaling in reprogramming CD8+ T cells differentiation.
CP-LNP1-Gal synergizes with αPD-1 to ameliorate liver metastatic outcomes by expanding TCF1+CX3CR1+ Tex subsets
Stem-like precursors with superior re-expansion capacity serve as the foundation for a robust response to ICB10. We hypothesized that the assembly of stem-like T cells induced by targeting hepatocytic-TβRI may synergize with ICB treatment to elicit a more robust CD8+ T cell response in liver metastasis. To achieve this, we developed choline-modified Lipid nanoparticles (CP-LNP1) as a nanoplatform for hepatocyte-specific drug delivery. We initially used the bioluminescence to monitor the organ-specificity of CP-LNP1, labeled with DID perchlorate for liposome tracking. As expected, CP-LNP1 exhibited significant liver accumulative fluorescence images compared to other major organs in both B16F10 tumor-bearing mice and control mice, peaking at 10 min and persisting for 24 h (Supplementary Fig. 9a). The cell-specificity of CP-LNP1 in the liver was assessed using immunofluorescence, revealing that DID fluorescence exclusively deposited in hepatocytes, confirming CP-LNP1 as a satisfactory hepatocyte-specific nanoplatform for drug delivery (Supplementary Fig. 9b, c).
Galunisertib was then directly loaded into the cholesterol-modified liposome, named CP-LNP1-Gal (Fig. 7a). Transmission electron microscopy confirmed the uniform spherical structure of CP-LNP1-Gal, with a particle size mainly in the range of 200–300 nm. Zeta potential results indicated that CP-LNP1-Gal is positively charged, with a potential of approximately + 20 Mv. High-performance liquid chromatography and ultraviolet spectroscopy were employed to detect the absorbance of Galunisertib at 254 nm and obtain the standard curve (Supplementary Fig. 9d). The drug loading capacity (DLC = 81.2%) was calculated as follows: DLC% = (drug loading capacity/weight of the entire nanoparticle) × 100 (Supplementary Fig. 9e). DID fluorescence imaging at various time points post-administration showed that CP-LNP1-Gal accumulated primarily in adjacent liver tissues rather than within the liver metastases and other organs (Fig. 7b). The immunofluorescence analysis revealed predominant localization of the DID signal in hepatocytes, with a minor deposition observed in macrophages, thereby confirming the targeted specificity of CP-LNP1-Gal towards hepatocytes (Fig. 7c–g). Additional immunofluorescence co-staining experiments using DID/p-Smad2/Alb/DAPI and DID/p-Smad2/F4/80/DAPI demonstrate that 24 h post-intervention, CP-LNP1-Gal treatment significantly reduces p-Smad2 expression in hepatocytes, while showing no apparent effect on p-Smad2 levels in macrophages (Fig. 7h, i). To evaluate its biological activity in vitro, AML12 cells were cultured with CP-LNP1-Gal. Remarkedly, CP-LNP1-Gal treatment notably neutralized the upregulated expression of p-Smad2/3 and Galectin-9 induced by TGFβ1(Supplementary Fig. 9f). Western blot analysis further demonstrated that CP-LNP1-Gal selectively attenuates TGFβ signaling activation in adjacent liver tissues, resulting in a significant reduction in Galectin-9 levels (Supplementary Fig. 9g). These results indicate that the hepatocyte-specific TGFβ signaling blocker CP-LNP1-Gal is effective in antagonizing hepatocytic Galectin-9 production.
a Schematic of CP-LNP1-Gal, a hepatocyte-targeting nano-liposome for TGFβ signaling inhibition, labeled with DID for in vivo tracking. Created in BioRender. Zhong, S. (2025) https://BioRender.com/qphmhxlb Representative ex vivo fluorescence images of various organ at indicated time points. Green dashed line: Liver metastasis; White dashed line: Normal liver. c–g Immunofluorescence analysis of DID localization within the liver metastatic microenvironment. LM: Liver metastasis; L: Normal Liver. The border between the liver metastatic lesion and the normal liver tissue is demarcated by a white dashed line. Scale bar, 20 μm. c Representative images of immunofluorescence co-staining for DID (pink) with Alb (green). d Representative images of immunofluorescence co-staining for DID (pink) with CD31 (green). e Representative images of immunofluorescence co-staining for DID (pink) with Desmin. f Representative images of immunofluorescence co-staining for DID (pink) with F4/80 (green). g Quantitative co-localization analysis using Pearson’s correlation coefficient. h, i Immunofluorescence co-staining for DID (pink) and p-smad2 (red) with Alb (green) or F4/80 (green) in mouse liver metastases. LM: Liver metastasis; L: Normal Liver. The border between the liver metastatic lesion and the normal liver tissue is demarcated by a white dashed line. Scale bar, 30 μm. h Representative images (Left) of immunofluorescence co-staining for DID (pink) and p-smad2 (red) with Alb (green). Quantification of p-smad2 in Alb-positive hepatocytes. i Representative images (Left) of immunofluorescence co-staining for DID (pink) and p-smad2 (red) with F4/80 (green). Quantification of p-smad2 in F4/80 positive macrophages. Data are presented as the mean ± SD, and were analyzed with one-way ANOVA with Sidak’s multiple comparison test (g) or unpaired two-sided Student’s t test (h, i). Consistent results were observed across three biological replicates in (b–i). Source data are provided as a Source Data file.
The in vivo antitumor efficacy and improvement in ICB resistance were subsequently evaluated in the B16F10 liver metastatic model (Fig. 8a). As expected, Galunisertib plus αPD-1 DID not significantly enhance tumor control compared with Galunisertib monotherapy, but the addition of αPD-1 improved the survival of mice treated with CP-LNP1-Gal and cured approximately 44.5% (4/9) of the mice. These results were validated through bioluminescence imaging and Log-rank survival analysis (Fig. 8b–d). Furthermore, we observed that CP-LNP1-Gal plus αPD-1 induced an unprecedented accumulation of TCF1+CX3CR1+ cells, constituting approximately 70% of the total responding CD8+ T cells. αPD-1 faithfully triggered the differentiation of TCF1+CX3CR1- cells assembled by CP-LNP1-Gal into TCF1+CX3CR1+ cells (Fig. 8e–i). In addition, we demonstrated that CP-LNP1-Gal similarly exhibits potent efficacy in controlling pancreatic cancer liver metastasis and synergizes with anti-PD1 therapy to achieve superior metastatic suppression (Supplementary Fig. 10a–d). These data support the idea that the hepatocytic-TβRI blocker CP-LNP1-Gal reverses resistance to PD-1 blockade and synergizes with immunotherapy in liver metastasis.
a Experimental setup to assess the effects of CP-LNP1-Gal or αPD-1 monotherapy and combination therapy in improving outcomes in B16F10-bearing C57BL/6 J mice. b Representative bioluminescent image of B16F10-bearing C57BL/6 J mice for indicated groups (n = 8 mice/group). c Quantification of bioluminescence in (b). d Survival curve of tumor-bearing mice for the indicated groups described in (b). Log-rank test for survival comparison. e Representative flow cytometry plots depicting the expression of TCF1 and CX3CR1 gated on CD8+PD1+CD44+ Tex in liver metastases for the indicated groups. f–i Statistical analysis of the percentages of TCF1+CX3CR1- (f), TCF1+CX3CR1+ (g), TCF1-CX3CR1+ (h), TCF1-CX3CR1- (i) subsets for indicated groups. Data are presented as the mean ± SD, and were analyzed with two-way ANOVA with Tukey’s multiple comparison test (c, f–i). Consistent results were observed across three biological replicates in (b–f). Source data are provided as a Source Data file.
Discussion
For the first time, we have identified a previously undescribed role for hepatocytes in facilitating liver metastasis. Our multiple preclinical liver metastatic models validate that conditional hepatocyte ablation of TβRI strikingly alleviates the burden of liver metastasis. Furthermore, in the PAN02-TGFβ1OE, PAN02-TGFβ1KD, or PAN02-TGFβ1CTL metastatic model, we observe that TβRI-hKO consistently provides protection from liver metastasis, regardless of the TGFβ1 level. These findings redefine the role of hepatocytes from bystanders to determinants that finely regulate the metastatic process. Emerging evidence suggests that tumor-derived TGFβ1 may initiate a feedforward loop by stabilizing TβRI through TGFBRAP1 binding, thereby suppressing its ubiquitin-mediated degradation31. However, the precise mechanisms by which the liver metastatic microenvironment orchestrates TβRI upregulation in hepatocytes remain elusive, necessitating further investigation into hepatocyte-tumor crosstalk and epigenetic regulators.
In this study, we observed a time-dependent expansion of Ly108⁺ stem-like exhausted CD8⁺ T cells in TβRI-hKO mice. This population increased significantly by day 12 post-implantation compared to TβRI-WT controls, whereas no notable differences were detected at earlier time points (Supplementary Fig. 11a, b). Furthermore, we visualize the existence and confirm the effector properties of the two Ly108+ subsets within the tumor metastatic microenvironment in the context of hepatocytic TGFβ signaling blockade. The tumor-control effects of these two subsets have not been described, according to our current knowledge. Specifically, our results describe a hybrid low-exhausted, intermediate-stem and high-effector state of Ly108+CX3CR1+ subset. Notably, although terminally differentiated Ly108-CX3CR1+ cells exhibit higher CD107a expression, which were reported to control virus in LCMV infection model32, their cytokine-producing capacity is limited in our metastatic models. Our results underscore TCF1-dominated stem-like traits are indispensable for CD8+ T-mediated anti-metastasis effect.
While the differentiation of progenitor Tex cells has conventionally been considered to follow a single linear path8, our lineage tracing experiments reveal two distinct lineage fates downstream of the Ly108+CX3CR1- precursors. As TCF1 progressively lost and Tox gradually accumulated, Ly108+CX3CR1- stem-like precursors underwent divergent differentiation trajectories, leading to the development of both Ly108-CX3CR1- terminally exhausted cells and Ly108+CX3CR1+ stem-like effector cells, with the latter subsequently transitioning into Ly108-CX3CR1+ terminal effector cells. However, it is crucial to note that our results do not preclude the possibility of naïve CD8+ T cells directly differentiating into the Ly108+CX3CR1+ subset. Furthermore, the transcriptional factor that mediates the bifurcation between these divergent fates remains to be thoroughly investigated. This discrepancy implies the existence of distinct Tex differentiation trajectories between virus infection and the tumor microenvironment.
Previous studies have shown that MHCII-expressing antigen-presenting cells are essential for the differentiation of stem-like CD8+ T cells in the tumor microenvironment33. In addition, reprogramming M2 macrophages to an M1 phenotype has been demonstrated to further support this differentiation, boosting immunotherapy efficacy34. Our findings extend this understanding by showing that targeting hepatocyte TGFβ signaling can independently promote the differentiation of stem-like CD8+ T cells, irrespective of macrophage involvement. This highlights an alternative pathway that reinforces the maintenance and functionality of stem-like CD8+ T cells in tumors, contributing to sustained anti-tumor immunity.
Contrary to previous studies emphasizing the positive correlation between increased numbers of recruited tumor-infiltrating lymphocytes (TILs) and enhanced efficacy of ICB and improved survival35, our findings suggest that TCF1-driven stem-like differentiation status is crucial for the anti-tumor response. Evidence has indicated that TCF1 is instrumental in ensuring the proliferative and reactive activity of CD8+ T cells36, thereby underpinning responses to ICB and vaccine therapy. Consequently, inducing appropriate TCF1 activity emerges as a promising target for immunotherapy.
Cytokines are absolutely required for regulating T cell proliferation and survival37,38. TGFβ-induced Galectin-9 secretion redirects the T cell differentiation streams towards terminal cells to impair the ongoing anti-tumor CD8+ T cell response. Mechanistically, Ly108+CX3CR1+ stem-like cells are the bona fide therapeutic T cells for Galectin-9 or TβRI blockade. Contrary to our expectation, the dual targeting strategy with TβRI blockade and Galectin-9 neutralization shows no synergistic effects. This outcome suggests that Galectin-9 acts as a nominated downstream signal molecule of TGFβ, and there exists a considerable overlap in the mechanistic regulation of CD8+ T cell between Galectin-9 and TGFβ. Hence, it is not surprising that the combination therapy only mildly enhanced antitumor therapeutic efficacy than that of Galunisertib monotherapy, without a significant difference.
Ongoing strategies to block TGFβ signaling predominantly encompass tumor-specific TGFβ-inhibitory therapies39 and combinatorial approaches involving TGFβ blockade and ICB40. A recent study by Chaurio41 demonstrated that TGFβ-mediated enhanced differentiation of human T cells into follicular helper T cells induced the assembly of intra-tumoral tertiary lymphoid structures, resulting in decreased tumor growth. Therefore, investigating cell-dependent responses in different cancer ecosystems is crucial for identifying accurate therapeutic targets for TGFβ inhibition. Our hepatocyte-targeted TGFβ blocker, CP-LNP1-Gal, synergizes with immunotherapy by potentiating effector-like differentiation without compromising the long-term persistence of precursor cells. Importantly, we show that systemic TGFβ inhibition with Galunisertib disrupts tertiary lymphoid structure neogenesis, whereas hepatocyte-targeted inhibition with CP-LNP1-Gal maintains high endothelial venule integrity (Supplementary Fig. 12a, b). This distinction underscores the therapeutic advantage of localized TGFβ blockade in promoting immune surveillance and sensitizing liver metastases to immunotherapy, opening new avenues for TGFβ-targeted treatment strategies.
Overall, our work highlights the critical role of hepatocytic TGFβ signaling in orchestrating the diversity of tumor-reactive T cell responses42. Within the liver’s pro-metastatic niche, hepatocytic TGFβ signaling emerges as a shared pathway employed by various tumors to establish an immunosuppressive microenvironment through the induction of terminal exhaustion in T cells. Targeting hepatocytic TβRI in advance represents a viable strategy for preserving an effective anti-tumor CD8+ T cell response. These findings illuminate the essential involvement of hepatocytes in reshaping the differentiation of CD8+ T cells and provide insights into leveraging TCF1-driven stem-like effector differentiation as a mean to control metastasis43.
Methods
Patients
Formalin-fixed paraffin-embedded human liver biopsy sections from patients were obtained from Guangdong Provincial People’s Hospital (Guangzhou, China). All samples were pathologically confirmed. Liver tissues were surgically obtained from thirty-eight patients with colorectal cancer with liver metastases and eight patients with colorectal cancer without liver metastases. This study has passed the ethics review of the Ethics Committee of Guangdong Provincial People’s Hospital, and the ethics number is KY2024-887-01. Patient information is presented in Supplementary Data 1. All patients provided a consent form, and all applicable regulations were followed.
Mice
TβRIflox/flox (Cat JAX: 028701), Alb-Cre+/+ (Cat JAX: 003574) mice were obtained from the Jackson Laboratory (USA). Galectin-9flox/flox (Cat S-CKO-03384) were obtained from Cyagen Biosciences (China), TβRIflox/flox mice were bred to Alb-Cre+/+ mice to generate TβRIflox/flox Alb-Cre+/- and TβRIflox/flox Alb-Cre+/+ mice (TβRI-hKO), or TβRIflox/flox Alb-Cre-/- mice (TβRI-hWT). Galectin-9flox/flox mice were bred to Alb-Cre+/+ mice to generate Galectin-9flox/flox Alb-Cre+/- and Galectin-9flox/flox Alb-Cre+/+ mice (Gal9-hKO), or Galectin-9flox/flox Alb-Cre-/- mice (Gal9-hWT). CD45.1 mice (Stock No: C001195) were purchased from Cyagen Biosciences (China). C57BL/6 J mice were purchased from Guangdong Medical Laboratory Animal Center (Stock No: 44826500002575). Both male and female mice were used in this study. Six- to eight-week-old age-matched mice were used in the experiments. All animals (4-5 mice per cage) were fed a regular diet and autoclaved water and were maintained under a 12 h light/dark cycle in a specific pathogen-free animal facility at a controlled temperature (21–24 °C) and humidity (50 ± 5%). All animal experiments were approved by the Ethical Committee for Experimental Animal Care of Southern Medical University (Guangzhou, China) (Resolution No: SMUL2021120). The studies reported in this paper are in accordance with the Animal Research: Reporting of In Vivo Experiments (ARRIVE) guidelines.
Cell lines
The mouse colon adenocarcinoma cell line MC38(1101MOU-PUMC000523), the mouse melanoma cell line B16F10 (1101MOU-PUMC000473) and the mouse pancreatic cancer cell line PAN02 (1101MOU-PUMC000446) were obtained from the Cell Resource Center, Peking Union Medical College (which is the headquarters of National Science & Technology Infrastructure—National Biomedical Cell-line Resource, NSTI-BMCR). Mouse hepatocyte cell line AML12 (CRL-2254) was purchased from ATCC. All cell lines were grown in complete DMEM-10 medium: DMEM (Gibco), 10% FBS (Gibco), 1% penicillin / streptomycin (GenXion) at 37 °C incubator with 5% CO2. Only cell lines that had been passaged fewer than 10 times were used for experiments, and trypan blue staining was used to ensure that cells with > 95% viability were used for studies. All cell lines used in our studies tested negative for Mycoplasma contamination.
Lentiviral construction and stable cell line generation
For bioluminescent imaging, MC38, PAN02 and B16F10 cells were transfected with CV146-Luciferase (Luc) reporter Lentiviral (Cat: CON285, GENECHEM, Shanghai), named MC38-Luc, PAN02-Luc and B16F10-Luc cells. All cells were selected with puromycin (Sigma Aldrich, 2 μg/mL).
For PAN02-Luc cells secreting active TGFβ1, CV557 vector encoding the biologically active form of mouse TGFβ1 (TGFβ1OE) was constructed by GENECHEM. Ubi-MCS-3FLAG-SV40-mCherry-IRES-neomycin mock vector (Cat: CON382, GENECHEM, Shanghai) was used as control. Infection efficiency was estimated under fluorescence microscope by the presence of mCherry-positive cells. Cells were selected with neomycin. The content of TGFβ1 in cell culture supernatants was confirmed by ELISA analysis (BD Bioscience, USA).
For in vitro knockdown of TGFβ1 in PAN02-luc cells, the custom-made GV654 lentiviral vectors encoding shRNAs with sequences targeting mouse TGFβ1 were purchased from GENECHEM (Shanghai, China). The specific targeting sequences including CGGCAGCTGTACATTGACTTT (TGFβ1KD1), GCTCTTGTGACAGCAAAGATA (TGFβ1KD2), AACAACGCCATCTATGAGAAA (TGFβ1KD3). No-targeting hU6-MCS-Ubiquitin-mCherry-IRES-Neomycin mock vector (Cat: CON464, GENECHEM, Shanghai) was used as a control. Infection efficiency was estimated under a fluorescence microscope by the presence of mCherry-positive cells. Cells were selected with neomycin. The content of TGFβ1 in cell culture supernatants was confirmed by ELISA analysis (BD Bioscience, USA). As the lowest TGFβ1 in cell culture supernatants was produced by 95191-lentiviral transfection in PAN02-Luc cells, we selected the 95191 targeting sequences to further experimentation.
Animal liver metastatic model
For intraportal injection of tumor cells, mice were anaesthetized using continuous isoflurane, and their abdomen was sterilized. After administration of analgesic agents, a 1- to 2 cm surgical incision was made on the median side of the mouse abdomen. After exposure of the peritoneal cavity, the intestines were located and exteriorized onto a sterile field surrounding the incision site to visualize the portal vein. Throughout the procedure, the intestines were kept hydrated with sterile PBS that was pre-warmed to 37 °C. For intraportal injection, PAN02-Luc (5 × 105 cells suspended in 100 μL sterile PBS), MC38-Luc cells (1 × 106 cells suspended in 100 μL sterile PBS) or B16F10-Luc cells (5 × 105cells (Fig. 1) or 2.5 × 105cells (Fig. 6) suspended in 100 μL sterile PBS) were injected into the portal vein via a 30-gauge needle. Successful injection was confirmed by partial blanching of the liver. After removal of the needle, bleeding was stopped by gently pressing the puncture site with a cotton swab combined with a small piece of an absorbable gelatin sponge. Afterwards, the intestines were placed back into the peritoneal cavity, and the peritoneum and skin were closed with a suture, respectively. All surgical procedures were performed in accordance with institutional guidelines.
Animal monitoring and ethical endpoints
This study was approved by the Ethics Committee of Southern Medical University (approval number: SMUL2021120), which permitted intraportal tumor transplantation, with a maximum permitted tumor burden not exceeding 1.5 cm in any dimension. Tumor size was monitored using small animal in vivo imaging, and euthanasia was performed when hunching or a reduction in spontaneous activity exceeding 50% was observed. Euthanasia was performed by intraperitoneal injection of 150 mg/kg sodium pentobarbital (No. P3761, Sigma), with the criterion for death being complete cessation of heartbeat and respiration.
Mouse treatments
For TAMs depletion in vivo, Clodronate Liposomes (YEASEN,40337ES10) or Control Liposomes (YEASEN,40338ES10) were injected intraperitoneally (5 mg/mL, 200 μL).
For CD8+ T cells depletion in vivo, Anti-mouse CD8a (BioXcell, BE0061) or rat IgG2b (BioXCell, BE0090) Isotype-control antibodies were diluted to 1 mg/mL in PBS, and 200 μL was administered per mouse by intraperitoneal injection.
For CD4+ T cells depletion in vivo, Anti-mouse CD4 (STARTER, S0B0690) or rat IgG2b (BioXCell, BE0090) Isotype-control antibodies were diluted to 1 mg/mL in PBS, and 200 μL was administered per mouse by intraperitoneal injection.
Galunisertib (Cat: S2230, Selleck) was suspended in 1% sodium carboxy methylcellulose (1% Na-CMC). Galunisertib or vehicle was administered by gavage in a 0.2 mL volume, 75 mg/kg, twice per day. For neutralization of Galectin-9, mice received 150 μg of αGal9 (RG9-1, BioXCell, BE0218) or rat IgG2b (LTF-2, BioXCell, BE0090) Isotype-control antibodies administration in 200 μL PBS.
For immune-checkpoint blockade experiments, Mice were intraperitoneally treated with 150 μg of anti-mouse PD-1 (RMP1-14, BioXcell, BE0146) or rat IgG2b (LTF-2, BioXCell, BE0090) Isotype-control antibodies.
CP-LNP1-Gal was injected into the tail vein at a dose of 75 mg/kg, 250 μL every two days starting three days after cell injection and ending 21 days after metastasis-initiation.
Bioluminescence imaging and analysis
Each mouse was injected with 1.5 mg D-Luciferin (Yeasen Biotechnology, Shanghai) intraperitoneally (100 μL) and imaged using IVIS Spectrum Imaging System (Perkin Elmer) after six-eight minutes. Images were acquired and analyzed by the Living Image 4.4 software (Perkin Elmer).
Tissue processing and preparation of single cell suspensions
After tumor challenge, T cells were isolated from spleen by cutting samples into small pieces, homogenizing against a 70 μm cell strainer and resuspended in 3 mL Red Blood Cell Lysis Buffer (Beyotime Biotechnology) for five minutes at room temperature (RT). Cell suspensions were washed twice with cold RPMI1640 medium with 2% FBS, 1% penicillin/streptomycin and resuspended in FACs Buffer (1% PBS, 2% FBS, 2 mM EDTA). Blood samples were collected in 2 ml PBS plus 100ul saturated EDTA-Na2 solution and enriched using Percoll (GE Healthcare) density gradient separation (30%/70%). Remaining red blood cells were lysed using Red Blood Cell Lysis Buffer (Beyotime Biotechnology) for five minutes at RT and processed as above. Liver metastatic tumors were mechanically disrupted and dissociated with scissors in 1 ml cold RPMI1640. Dissociated tissue pieces were digested for one hour at 37 °C in serum-free RPMI 1640 medium supplemented with 1 mg/ml collagenase IV (Gibco), 1 mg/ml collagenase I (Gibco), 0.02 mg/mL DNase I (Sigma-Aldrich) and 0.1 mg/ml Hyaluronidase (Sigma-Aldrich). Digestion was quenched by adding 1 ml FBS. Cell suspensions were further disrupted with the back of a 3 ml syringe, filtered through a 70-μm strainer and washed twice with FACs Buffer. After red blood cells were lysed in Red Blood Cell Lysis Buffer (Beyotime Biotechnology) for 5 min and samples were refiltered through a 70 μm strainer, lymphocytes from the tumor were enriched using Percoll (GE Healthcare) density gradient separation (40%/80%), washed two times with cold RPMI and counted using a hemocytometer for absolute number calculations.
To assess cytokine and effector molecule production, CD8+ T cells were enriched from total lymphocytes using the mouse CD8+ (TIL) Micro-Beads (Miltenyi Biotec) and counted using a hemocytometer for absolute number calculations. For ex vivo cytokine expression analysis, 1 × 106 CD8+ T cells were plated in 500 μL RPMI in wells of a flat-bottom 24-well dish and stimulated with Leukocyte Activation Cocktail with BD GolgiPlug (BD Biosciences) for 5 h at 37 °C. For ex vivo effector molecule production analysis, 1 × 106 CD8+ T cells were plated in 500 μL RPMI in wells of a flat-bottom 24-well dish and stimulated with 1 μg/mL CD3 mAb and 5 μg/mL CD28mAb (BD Biosciences), BD GolgiPlug and BD GolgiStop for 5 h at 37 °C.
Flow cytometry
Equal number of cells were prepared from various tissues and were first stained with Fixable Viability Dye 620 (BD Biosciences) for 15 min at 4 °C in the dark to exclude non-viable cells. For tumor samples staining, Live cells were stained with extracellular antibodies for 30 min on ice in the presence of anti-CD16/32 (BD Biosciences). For intracellular staining of cytokines, the Cytofix/Cytoperm Fixation/Permeabilization Solution Kit from BD Biosciences was used as per the manufacturer’s protocol. For intracellular staining, the FoxP3/Transcription Factor staining buffer set (eBioscience) was used as per the manufacturer’s protocol. The following antibodies were used in the analysis: BD Bioscience: CD45, CD4, CD8, CD44, KLRG1, 2B4, LAG-3, TIGIT, Ly108, CD107a, T-bet, TNF, IL2. Thermo Fisher Scientific: Tim-3, Granzyme B, Eomes, CD39. BioLegend: PD-1, CX3CR1, IFNγ, CD127, CD3, Ki-67. R&D Systems: TCF1. Miltenyi Biotec: TOX. All flow cytometry antibodies were used at a dilution of 1:100. Cells were resuspended in FACS buffer and acquired on a CytoFLEX S flow cytometer (BECMAN COULTER Life Sciences). Datasets were analyzed using FlowJo software (FlowJo, LLC).
ELISA
ELISA test kits were used to detect TGFβ1 (BD Bioscience) and Galectin-9 (RayBiotech) in cell supernatant and mouse tumor interstitial fluid. We used a microplate reader (Multiskan; Thermo Fisher Scientific) to measure the absorbance at 450 nm or 570 nm.
Western blot Analysis
Cells were lysed in the cell lysis buffer (Beyotime) containing 1 mM phenylmethylsulfonyl fluoride and a protease inhibitor cocktail. The protein concentrations were determined by a BCA kit (Thermo Fisher Scientific). Then, samples (30 µg protein/lane) were electrophoresed in SDS-PAGE and transferred to a Polyvinylidene Fluoride membrane. The membrane was blocked with 5% bovine serum albumin and subsequently incubated overnight at 4 °C with Galectin 9 (1:1000, Abcam), p-smad2 (1:1000, Abcam), GAPDH (1:1000, CST). This was followed by incubation with horseradish peroxidase (HRP) conjugated secondary antibodies. The specific protein bands were visualized using an enhanced ECL system (Bio-Rad).
Luminex multiplex assays
The protein levels of inflammatory cytokines in tumor interstitial fluid of TβRI-hWT and TβRI-hKO mice were analyzed by Luminex multiplex assays (LabEX, UNIV, Shanghai).
Cell sorting and adoptive T cells transfer
Ly108+CX3CR1- and Ly108+CX3CR1+ cells were sorted from TβRI-hKO mice inoculated with PAN02 in the portal vein on day 15. Ly108-CX3CR1+ and Ly108-CX3CR1- cells were sorted from C57BL/6 J mice inoculated with PAN02 in the portal vein on day 15. CD45.1+ recipient mice were intraperitoneally administrated with 4 mg cyclophosphamide (CTX, Sigma, PHR1404) at 6–8 days after inoculating with PAN02 in the portal vein. For lineage analysis, 2 × 105 cells were transferred into CD45.1+ recipients, respectively. To assess liver metastasis repressive capacity from different subsets, 5 × 105 cells were transferred into CD45.1+ recipients, respectively. 15 days later, CD8+PD1+CD44+CD45.2+CD45.1- cells were sorted from each transferred mouse to envaulted the expression of Ly108 and CX3CR1.
Bone marrow cells were isolated from the tibia and femur of 6–8-week-old CD45.1 mice and differentiated in the presence of M-CSF (10 ng/ml) in RPMI-1640 growth medium. On day 5, The BMDM and PAN02 cell lines were co-injected into the portal vein of CD45.2 mice at a 1:1 ratio.
Immunohistochemistry (IHC) staining and multicolor IHC staining
Immunohistochemical staining was conducted according to the manufacturer’s protocol. Briefly, the slides were heated at 60 °C for 2 h, deparaffinized in xylene, rehydrated with 100%, 90%, 80% and 70% ethanol, and immersed in methanol with 3% H2O2 (hydrogen peroxide) for 15 min at room temperature to inactivate endogenous peroxidase. Antigens were heat-retrieved in sodium citrate buffer (10 mM sodium citrate and 0.05% Tween 20, pH 6.0) at 100 °C for 8 min. After blocked with 10% serum for 1 h at room temperature, the slides were incubated with primary antibodies against TGFBR1/P-smad2/α-SMA/HNF4α/PAN-CK/CD68/Galectin9 over nights at 4 °C. The second day, the slices were incubated with secondary antibodies (Gene tech) for 30 min at room temperature, the sections were stained with DAB and counterstained in hematoxylin. After dehydration and coverslip, images were captured under a microscope (Digital Pathology Slide Scanner) using a program. Multicolor immunohistochemistry staining “Multiplex fluorescence immunohistochemical kit” (ABSIN, Cat: mIHC-1274-6-EA) was used. Dilution ratios for antibodies have been added to Supplementary Data 2.
Immunofluorescence (IF) staining
The slides were heated at 60 °C for 2 h, deparaffinized in xylene, rehydrated with 100%, 90%, 80% and 70% ethanol, and immersed in methanol with 3% H2O2 (hydrogen peroxide) for 15 min at room temperature to inactivate endogenous peroxidase. Antigens were heat-retrieved in sodium citrate buffer (10 mM sodium citrate and 0.05% Tween 20, pH 6.0) at 100 °C for 8 min. After blocked with 10% serum for 1 h at room temperature, the slides were incubated with primary antibodies against TGFBR1/P-smad2/Desmin/CD31/F4-80/Alb/Galectin9 over nights at 4 °C. After washing three times with PBS, the cells were stained with secondary antibodies. Nuclei were counterstained with DAPI. Images were captured using a laser confocal microscope (NIS-Elements Viewer). Dilution ratios for antibodies have been added to Supplementary Data 2.
Preparation and characterizations of the nanoparticles
CP-LNP1-Gal was prepared by ethanol injection. Firstly, the choline polymer (P1) 2.15 mg, cholesterol (Chol) 1.25 mg, distearyl phosphatidylcholine (DSPC) 9 mg, DID perchlorate 0.1 mg, and Galunisertib 1.6 mg were accurately weighed and dissolved in 1 mL anhydrous ethanol. Add to 9 mL phosphate buffer (PBS) under the condition of rapid agitation and stir for 1 min; It was then transferred to a dialysis bag (MWCO = 7000 Da) and subjected to photodialysis in PBS (10 mM, pH 7.4) solution for 12 h. The PBS solution was changed every 4 h to remove the organic solvent.
The particle size distribution was measured using a Malvern Zeta-sizer Nano ZS instrument. Transmission electron microscope (TEM) measurements were conducted on a JEOL 2010 electron microscope. Reversed-phase HPLC (RP-HPLC) analysis was performed on a LC-20A HPLC system (Shimadzu), and calculated by the following formulae:
Statistical analysis
Data are presented as mean ± SD. Two-sided unpaired Student’s t test, one-way ANOVA with Tukey’s or Sidak’s multiple comparison test, two-way ANOVA with Tukey’s or Sidak’s multiple comparison test were used when appropriate and as indicated. All the P values are directly indicated on the statistical graph. All statistical analyses were performed using GraphPad Prism v.8.3.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
All data are included in the Supplementary Information or available from the authors, as are unique reagents used in this Article. The raw numbers for charts and graphs are available in the Source Data file whenever possible. Source data are provided in this paper.
Materials availability
All materials are readily available from the indicated standard commercial sources.
References
Horn, S. R. et al. Epidemiology of liver metastases. Cancer Epidemiol. 67, 101760 (2020).
Gottwick, C., Carambia, A. & Herkel, J. Harnessing the liver to induce antigen-specific immune tolerance. SDinars Immunopathol. 44, 475–484 (2022).
Lee, J. C. et al. The liver-immunity nexus and cancer immunotherapy. Clin. Cancer Res. 28, 5–12 (2022).
Gerstberger, S., Jiang, Q. & Ganesh, K. Metastasis. Cell 186, 1564–1579 (2023).
Correia, A. L. et al. Hepatic stellate cells suppress NK cell-sustained breast cancer dormancy. Nature 594, 566–571 (2021).
Pauken, K. E. et al. Epigenetic stability of exhausted T cells limits durability of reinvigoration by PD-1 blockade. Science 354, 1160–1165 (2016).
McLane, L. M., Abdel-Hakeem, M. S. & Wherry, E. J. CD8 T Cell exhaustion during chronic viral infection and cancer. Annu. Rev. Immunol. 37, 457–495 (2019).
Chen, Z. et al. TCF-1-Centered transcriptional network drives an effector versus exhausted CD8 T cell-fate decision. Immunity 51, 840–855e845 (2019).
Khan, O. et al. TOX transcriptionally and epigenetically programs CD8( + ) T cell exhaustion. Nature 571, 211–218 (2019).
Zehn, D., Thimme, R., Lugli, E., de Almeida, G. P. & Oxenius, A. Stem-like’ precursors are the fount to sustain persistent CD8( + ) T cell responses. Nat. Immunol. 23, 836–847 (2022).
Scott, A. C. et al. TOX is a critical regulator of tumour-specific T cell differentiation. Nature 571, 270–274 (2019).
Mariathasan, S. et al. TGFβ attenuates tumour response to PD-L1 blockade by contributing to exclusion of T cells. Nature 554, 544–548 (2018).
Guinney, J. et al. The consensus molecular subtypes of colorectal cancer. Nat. Med. 21, 1350–1356 (2015).
Zhao, L. et al. Promotion of colorectal cancer growth and metastasis by the LIM and SH3 domain protein 1. Gut 59, 1226–1235 (2010).
Zhang, F. et al. MicroRNA-187, a downstream effector of TGFbeta pathway, suppresses Smad-mediated epithelial-mesenchymal transition in colorectal cancer. Cancer Lett. 373, 203–213 (2016).
Tauriello, D. V. F. et al. TGFbeta drives immune evasion in genetically reconstituted colon cancer metastasis. Nature 554, 538–543 (2018).
McKinney, E. F., Lee, J. C., Jayne, D. R., Lyons, P. A. & Smith, K. G. T-cell exhaustion, co-stimulation and clinical outcome in autoimmunity and infection. Nature 523, 612–616 (2015).
Wang, Y. et al. Targeting Src SH3 domain-mediated glycolysis of HSC suppresses transcriptome, myofibroblastic activation, and colorectal liver metastasis. Hepatology 80, 578–594 (2024).
Hudson, W. H. et al. Proliferating transitory T cells with an effector-like transcriptional signature emerge from PD-1(+) stem-like CD8( + ) T cells during chronic infection. Immunity 51, 1043–1058 (2019).
Zander, R. et al. CD4( + ) T Cell help is required for the formation of a cytolytic CD8( + ) T cell subset that protects against chronic infection and cancer. Immunity 51, 1028–1042 (2019).
Im, S. J. et al. Defining CD8 + T cells that provide the proliferative burst after PD-1 therapy. Nature 537, 417–421 (2016).
Daniel, B. et al. Divergent clonal differentiation trajectories of T cell exhaustion. Nat. Immunol. 23, 1614–1627 (2022).
Brunner, A. M., Marquardt, H., Malacko, A. R., Lioubin, M. N. & Purchio, A. F. Site-directed mutagenesis of cysteine residues in the pro region of the transforming growth factor β1 precursor. J. Biol. Chem. 264, 13660–13664 (1989).
Mulder, K. et al. Cross-tissue single-cell landscape of human monocytes and macrophages in health and disease. Immunity 54, 1883–1900 (2021).
Shin, A. E. et al. F4/80(+)Ly6C(high) Macrophages lead to cell plasticity and cancer initiation in colitis. Gastroenterology 164, 593–609.e513 (2023).
Golden-Mason, L. & Rosen, H. R. Galectin-9: Diverse roles in hepatic immune homeostasis and inflammation. Hepatology 66, 271–279 (2017).
Yang, R. et al. Galectin-9 interacts with PD-1 and TIM-3 to regulate T cell death and is a target for cancer immunotherapy. Nat. Commun. 12, 832 (2021).
Wu, C. et al. Galectin-9-CD44 interaction enhances stability and function of adaptive regulatory T cells. Immunity 41, 270–282 (2014).
Elahi, S., Niki, T., Hirashima, M. & Horton, H. Galectin-9 binding to Tim-3 renders activated human CD4 + T cells less susceptible to HIV-1 infection. Blood 119, 4192–4204 (2012).
Zhuang, J. et al. TGFβ1 Promotes gemcitabine resistance through regulating the LncRNA-LET/NF90/miR-145 signaling axis in bladder cancer. Theranostics 7, 3053–3067 (2017).
Liu, K. et al. Stabilization of TGF-beta receptor 1 by a receptor-associated adaptor dictates feedback activation of the TGF-beta signaling pathway to maintain liver cancer stemness and drug resistance. Adv. Sci. 11, e2402327 (2024).
Buchholz, V. R. & Busch, D. H. Back to the future: effector fate during T cell exhaustion. Immunity 51, 970–972 (2019).
Jansen, C. S. et al. An intra-tumoral niche maintains and differentiates stem-like CD8 T cells. Nature 576, 465–470 (2019).
Zhang, X. et al. Cell microparticles loaded with tumor antigen and resiquimod reprogram tumor-associated macrophages and promote stem-like CD8( + ) T cells to boost anti-PD-1 therapy. Nat. Commun. 14, 5653 (2023).
Siddiqui, I. et al. Intratumoral Tcf1(+)PD-1( + )CD8( + ) T cells with stem-like properties promote tumor control in response to vaccination and checkpoint blockade immunotherapy. Immunity 50, 195–211 (2019).
Shan, Q. et al. Tcf1-CTCF cooperativity shapes genomic architecture to promote CD8( + ) T cell homeostasis. Nat. Immunol. 23, 1222–1235 (2022).
Liu, Y. et al. IL-2 regulates tumor-reactive CD8( + ) T cell exhaustion by activating the aryl hydrocarbon receptor. Nat. Immunol. 22, 358–369 (2021).
Ozga, A. J. et al. CXCL10 chemokine regulates heterogeneity of the CD8 + T cell response and viral set point during chronic infection. Immunity 55, 82–97 (2022).
DePeaux, K. et al. An oncolytic virus-delivered TGFβ inhibitor overcomes the immunosuppressive tumor microenvironment. J. Exp. Med. 220, (2023).
Massagué, J. & Sheppard, D. TGF-β signaling in health and disease. Cell 186, 4007–4037 (2023).
Chaurio, R. A. et al. TGF-β-mediated silencing of genomic organizer SATB1 promotes Tfh cell differentiation and formation of intra-tumoral tertiary lymphoid structures. Immunity 55, 115–128 (2022).
Bald, T. & Smyth, M. J. TGFβ shuts the door on T cells. Br. J. Cancer 119, 1–3 (2018).
Escobar, G. et al. Tumor immunogenicity dictates reliance on TCF1 in CD8( + ) T cells for response to immunotherapy. Cancer Cell 41, 1662–1679 (2023).
Acknowledgements
The authors express their gratitude to Xueqing Yao’s team from the Department of Gastrointestinal Surgery at Guangdong Provincial People’s Hospital for their invaluable assistance in the collection and follow-up of clinical samples in this study. This work was supported by the National Natural Science Foundation of China (82574892, 82274387, 82405215), the National Key Research and Development Program of China (2023YFC3502802, 2025ZD0545102), the Research and Development Program of Department of Education of Guangdong province (2022ZDZX2008), the Key Research and Development Program of Guangzhou Municipal Science and Technology Bureau (SL2023B03J00735).
Author information
Authors and Affiliations
Contributions
Conceptualization: X.S. and H.W.; Methodology: H.W., J.Y., K.Y., B.G., and J.W.; Data curation: H.W., Y.Z. B.G., X.Y., Y.T., and S.Z.; Investigation: X.S., H.W., C.H., Q.Z., X.W., and J.W.; Resources: J.Y., Y.Z., B.G., Y.D., Y.M., S.Z., and Q.H.; Writing – Original Draft: H.W.; Review & Editing: X.S., L.Z., and X.W.; Project administration: X.S.; Supervision: X.S. and L.Z.; Funding acquisition: H.W. and X.S.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Wang, H., Zhou, Y., Tu, Y. et al. Targeting hepatocytic TβRI ameliorates liver metastatic outcomes by revitalizing stem-like CD8+ Tex subsets. Nat Commun 16, 10627 (2025). https://doi.org/10.1038/s41467-025-65615-0
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41467-025-65615-0