Abstract
Immune cell functions are dictated by their differentiation state and regulated by transcriptional and epigenetic changes. Immune cell differentiation also controls the preferential metabolic pathways used for energy production. However, whether the energy charge of individual immune cells itself varies across time and space and regulates cell function remains to be fully understood. Here, we show that T cells harbor distinct energetic resources and function in different anatomical locations and times of the day. To monitor ATP: ADP ratio, an indicator of cellular energetic resources, we rely on SPICE-Met, a method that dissects energy metabolism in complex cell populations in vivo. We find that cells with the highest glycolytic capacity, including effector T cells and NK cells, exhibit the highest ATP: ADP ratio. Importantly, effector T cells but not naïve T cells display higher energetic charge when present in the blood compared to lymph nodes due to differential glucose availability. Energetic resources are also regulated in a circadian manner, being highest at the early rest phase. Importantly, differences in energetic charge are directly translated at the level of T cell function, impacting IFN-γ production. Thus, modulation of energetic charge and nutrient availability dictates immune cell function across time and space.
Similar content being viewed by others
Introduction
Immune cells exert a variety of effector functions based on their differentiation state, regulated by transcriptional and epigenetic changes. In addition, cell differentiation is also often associated with unique metabolic profiles, including specific metabolic pathways used for energy production1. For example, naïve or central memory T cells largely rely on OXPHOS while effector T cells primarily use glycolysis for energy production2. Within the cell, energy relies on generation of ATP, a key molecule used to store chemical energy and fuel a variety of cellular processes. Upon hydrolysis, ATP is converted to ADP, and energy is released3. Therefore, the cellular ATP:ADP ratio reflects the capacity of the cell to maintain its energetic resources and can be considered as a proxy for the cell energetic status or charge4,5,6. It remains, however, unclear whether a given immune cell, once differentiated, displays substantial changes in energetic charge during its life span, due, for example, to variations in nutrient availability. In fact, even at homeostasis, immune cells are likely to experience substantial nutrient fluctuations. For example, many immune cells, including T cells, migrate between secondary lymphoid organs, blood, and peripheral tissue7, which may each offer specific nutrient niches. Moreover, the niches themselves can potentially be subjected to nutrient oscillations due to circadian changes in physiology8. How changes in nutrient exposure across time and space impact the cellular energetic charge and function of immune cells remains to be explored.
Addressing these questions remains challenging. While a variety of approaches can provide information on immunometabolism9, few are dedicated to studying the cellular energetic charge and dependence on metabolic pathways and nutrients. We recently described SPICE-Met, a methodology to simultaneously profile energy metabolism in complex populations, providing single-cell information on ATP:ADP ratio and dependence in metabolic pathways and nutrients10. SPICE-Met is based on a transgenic mouse (SPICY mice) expressing a fluorescent reporter for ATP:ADP ratio11 in immune cells and the use of specific metabolic inhibitors10. SPICE-Met give results comparable to SeaHorse for example, regarding energy metabolic pathways, but can be performed on multiple defined subpopulations without cell sorting and with minimal sample processing time10.
Here, we uncovered that the energetic charge of effector T cells is a dynamic parameter, influenced by anatomical site, nutrient availability, and circadian time. Changes in energetic status across time and space critically impacted effector functions, revealing a new parameter tuning immune responses.
Results
Evaluating the relative and net contribution of metabolic pathways and substrates on cellular energetic resources
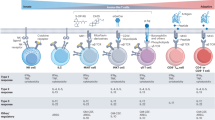
ATP:ADP ratio is an essential parameter of cellular energy status4. To explore how energetic resources impact immune cell function, we relied on SPICE-Met10, a method that used transgenic mice (SPICY mice) expressing the PercevalHR11 fluorescent reporter for ATP:ADP ratio. Competitive binding of ATP and ADP to the probe is reflected by changes in fluorescence signals collected by excitation at two different wavelengths, yielding an estimated value for the cellular energetic charge. These ratiometric measurements are therefore largely independent of the probe expression level. When used in flow cytometry, these mice allow for direct measurement of energetic resources in multiple cell populations simultaneously (Fig. 1A). Moreover, changes in ATP:ADP reporter activity upon treatment with metabolic inhibitors reveal dominant pathways and substrates contributing to energy production. For example, changes in ATP:ADP ratio upon treatment with oligomycin (which blocks mitochondrial respiration) reflect the dependence on OXPHOS versus glycolytic capacity (Fig. 1B). Similarly, treatment with iodoacetic acid or 2-DG (which block glucose metabolism) reveal cell dependence on glucose versus alternative energy substrates (amino-acids, or fatty acids) (Fig. 1C). Drops in ATP:ADP ratio upon inhibitor treatment can be expressed as percentage of total energy reserve (relative contribution) (Fig. 1B, C). Since ATP:ADP ratio may vary between cell types, it is important to consider how individual pathways quantitatively contribute to the raw value of ATP:ADP ratio, and we referred to these values as net contributions.
Pipeline for SPICE-Met analyses: a method that uses transgenic mice expressing a fluorescent reporter for ATP:ADP ratio to profile energy metabolism in complex cell populations. A Experimental scheme. Immune cell populations were isolated from blood of Vav-Cre/Percevalfl/fl (SPICY) mice, and metabolic parameters were addressed by SPICE-Met flow cytometry. B Oligomycin (Oligo) treatment was used to reveal OXPHOS/glycolysis relative and net contributions to the generation of cellular energy resources. C Iodoacetic acid (IA) treatment was used to reveal glucose/fatty, amino acids, relative and net contributions into the generation of cellular energy resources. D–F Diversity of ATP:ADP ratio and energy metabolism in various immune cell populations from the blood. D ATP:ADP ratio measured for the indicated populations. E Heatmaps representing the net and relative contribution (dependency) of OXPHOS, glycolysis, glucose, and FA/AA for the indicated cell population. F Quantification of the net contribution of OXPHOS, glycolysis, glucose and FA/AA for the indicated cell population. Compiled from 21 independent experiments with a total of 24 mice. G Correlation analysis of ATP:ADP ratio of blood lymphocytes with different metabolic parameters. Compiled from 21 independent experiments with a total of 24 mice. Each dot represents the value obtained in one mouse.
Diversity of energy metabolism of immune cells in the blood
We rely on this methodology to profile energetic resources in immune cells in the blood. Interestingly, we observed extensive variability in cellular ATP:ADP ratio among leukocyte populations with the highest values observed in B cells, neutrophils, and effector lymphocytes (T cells, NK cells and NKT cells) (Fig. 1D). By contrast, quiescent T cells (naive and central memory) exhibit lower ATP:ADP ratios. Next, we assessed the link between ATP:ADP ratios and metabolic pathways and substrates (Fig. 1E, F). Overall, we found that immune cells with the highest ATP:ADP ratios exhibited elevated net contribution of glycolysis and glucose metabolism. As expected, naive and central memory T cells almost exclusively relied on OXPHOS (high relative contribution) but the net contribution of OXPHOS to the ATP:ADP value remained similar (if not lower) to that seen in effector cells. This observation highlights the importance of measuring changes in ATP:ADP ratio both qualitatively (relative contribution) and quantitatively (net contribution). In search for possible correlation between ATP:ADP ratio and other metabolic parameters, we found that the net (but not the relative) contribution of glycolysis was best correlated (Fig. 1G).
In sum, we revealed extensive differences in ATP: ADP ratio of coexisting immune cells in the blood that originated for distinct quantitative input of metabolic pathways and nutrients.
Anatomical regulation of lymphocyte energetic resources
Most lymphocyte populations can recirculate between the blood, lymphoid organs and/or peripheral tissues. Yet, the extent to which energetic resources of individual immune cells are intrinsically determined or influenced by their anatomical location is unclear.
To address this question, we compared energetic status and metabolism of lymphocyte populations, isolated from blood and lymph nodes (LN) that both contain numerous lymphocyte subsets (Fig. 2A). While naïve and central memory T cells exhibited similar ATP:ADP ratio in both locations, we observed that the cellular energetic charge of effector lymphocyte populations including CD8+ effector T cells, NK and NKT cells were remarkably higher in the blood (Fig. 2B, C).
A–C Immune cell populations were isolated from blood and lymph nodes of Vav-Cre/Percevalfl/fl (SPICY) mice, and metabolic parameters were addressed by SPICE-Met flow cytometry. A Experimental scheme. B Heatmaps representing the net contribution (dependency) of OXPHOS, glycolysis, glucose and FA/AA for the indicated cell population. C Representative FACS profile showing Perceval-ATP and Perceval-ADP fluorescence and ATP:ADP ratio in CD8+ effector/effector memory T cells and CD8+ central memory T cells (left) and quantification of ATP:ADP ratio in the indicated populations (right). D–F Vav-Cre/Percevalfl/fl (SPICY) mice were injected with MVA-vaccine and 7 days later, tetramer-positive cells were obtained from blood and lymph nodes, and their metabolic parameters were addressed. D Experimental scheme. E Representative FACS profile showing ATP and ADP fluorescence and ATP:ADP ratio in MVA-specific CD8+ effector/effector memory-like T cells and MVA-specific CD8+ central memory-like T cells. F Heatmaps and quantification of metabolic parameters of MVA-specific CD8+ effector/effector memory T cells and MVA-specific CD8+ central memory T cells. Compiled from 2 independent experiments with a total of 6 mice. Mean values ± SEM are shown. Statistical analyses were performed using a two-sided Wilcoxon test (C, F).
A deeper look into their metabolism revealed that effector T cells, NK, and NKT cells displayed a higher net contribution of glycolysis in the blood compared to LNs, and to a lower extent, an increase in the net contribution of OXPHOS. By contrast, the contribution of metabolic pathways in naïve T cells remained relatively similar between the blood and LNs (Fig. 2B, C).
To extend our results to an ongoing antigen-specific T cell response, we relied on a vaccine setting12 using the MVA vector (Fig. 2D). Briefly, mice were injected with MVA-based vaccine, and the ATP:ADP ratio was measured among MVA-specific effector-like (CD44+CD62L-) and central memory-like (CD44+CD62L+) CD8+ T cells at day 7. Reminiscent of what we observed at steady-state, antigen-specific T cells with an effector phenotype (but not central memory-like T cells) showed increased ATP:ADP ratio in the blood, compared to the LNs (Fig. 2E, F). These changes were accompanied by increased glycolysis, OXPHOS, and nutrient dependencies (Fig. 2E-F). Together, these data revealed that lymphocytes with similar phenotypes can possess different energetic charges in distinct anatomical locations.
Tissue microenvironment dictates T cell energetic resources
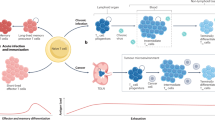
The different energetic resources observed for effector/effector memory T cells (CD44+CD62L-) present in the blood and lymph nodes could possibly be explained by preferential homing of distinct T cell subpopulations. In this case, an energetic resource would be an intrinsic property of T cells. Alternatively, a given T cell may harbor different energetic charge upon relocation in different anatomical sites. Under this hypothesis, the tissue microenvironment primarily dictates ATP:ADP ratio. To distinguish between these possibilities, we assess how energetic resources of effector T cells isolated from lymph nodes evolve upon adoptive transfer, as these cells redistribute between blood and lymph nodes of the recipient (Fig. 3A). We measured metabolic parameters from transferred lymph node cells in the blood and lymph nodes of the host as early as 3 hours after the transfer (Fig. 3B). Strikingly, effector CD4+ T and CD8+ T cells showed increased ATP:ADP ratio in the blood as compared to lymph nodes together with increased contribution of OXPHOS and glycolysis (Fig. 3C, D). On the other hand, transferred naïve CD4+ and CD8+ T cells maintained the same ATP:ADP ratio upon redistribution in the blood and lymph nodes, in good agreement with steady state results (Fig. 3C, D). Thus, the tissue microenvironment dictates cellular energetic resources in effector T cells.
A Experimental scheme. Total immune cell populations from lymph nodes of Vav-Cre/Percevalfl/fl (SPICY) mice and injected into CD45.1 recipients. CD45.2-positive cells were isolated 3 h later from the blood and lymph nodes of recipient mice and their metabolic parameters were addressed. B Detection of CD45.2-positive cells isolated from CD45.1 recipients 3 h after the adoptive transfer. C Representative FACS plot showing the ATP and ADP fluorescence and ATP:ADP ratio in the indicated populations within CD45.2-positive cells detected in the blood and lymph nodes of recipient mice. D Heatmaps and quantification of energetic resources and metabolic parameters in the indicated populations within CD45.2-positive cells detected in the blood and lymph nodes of recipient mice. Mean values ± SEM are shown. Compiled from 3 independent experiments with a total of 6 recipient mice. Statistical analyses were performed using a two-sided Wilcoxon test.
Glucose availability contributes to the anatomical regulation of immune cell energetic resources
As glycolysis and glucose consumption a hallmarks of effector T cells (Fig. 1E), we tested whether glucose concentration could be a key microenvironmental parameter that accounted for the observed differences in the ATP:ADP ratio measured in the blood and lymph nodes. It has been shown that immune cells upregulate the glucose transporter GLUT1 in glucose-poor microenvironment13,14,15,16. We therefore evaluated the expression of GLUT1 in blood and LN lymphocytes (Fig. 4A). Interestingly, for all tested lymphocyte populations including T cells, NK cells and NKT cells, GLUT1+ cells were barely detectable in the blood. By contrast, GLUT1+ cells were readily found in lymph nodes, representing for example, up to 40% of CD8+ effector T cells (Fig. 4A–C). Similar results were obtained using 3 distinct anti-GLUT1 Abs (Supplementary Fig. 1). Consistent with the idea the GLUT1 expression is upregulated under energetic stress, we observed an overall decreased ATP:ADP ratio in GLUT1+ cells as compared to the GLUT1- counterpart (Fig. 4D–F). Consistently GLUT1+ CD8+CD44+CD62L-effector/effector memory T cells showed low mTOR activity (Supplementary Fig. 2A, B). On the other hand, adoptively transferred GLUT1+ effector CD8+ T cells exhibited increased intracellular glucose (Supplementary Fig. 2C). These results raise the possibility that lymphocytes in the LNs microenvironment are subjected to metabolic stress, leading to upregulation of GLUT1 to overcome glucose deprivation. Of note, naïve T cells only modestly upregulated GLUT1 in the lymph node (Fig. 4C), consistent with their low dependence on glucose for energy production (Fig. 1E, F). To directly assess glucose concentration within immune cells located either in the blood or lymph nodes, we employed a recently developed glucose reporter iGluco17 that changes fluorescent spectrum upon glucose binding. Specifically, we transferred activated T cells (containing both CD4+ and CD8+ cells) transduced with iGluco probe and monitored iGluco activity upon their relocation in the blood or lymph nodes (Fig. 4G, H). We found that T cells in the blood exhibited a higher intracellular glucose concentration compared to lymph nodes (Fig. 4I). Moreover, we noted that iGluco fluorescence was directly reflecting glucose concentration in the extracellular milieu, supporting the idea that the LN microenvironment is glucose-poor compared to the blood.
A–C Immune cell populations were isolated from blood and lymph nodes of C57BL/6J mice, and percentage of GLUT1-positive cells were determined. Heatmaps (A), representative FACS plots (B) and percentage (C) of GLUT1+ cells in the indicated populations. Statistical analyses were performed using a two-sided Wilcoxon test. Compiled from 2 independent experiments with a total of 6 mice. D–F Immune cell populations were isolated from blood and lymph nodes of Vav-Cre/Percevalfl/fl (SPICY) mice, and energetic charge of GLUT1-positive and negative populations was measured in each cell population. Heatmaps (D), Representative FACS plots (E) and quantification (F) of ATP:ADP ratio within GLUT1+ and GLUT1- subsets. Statistical analyses were performed using a two-sided Wilcoxon test. Compiled from 2 independent experiments with a total of 6 mice. G–I Glucose concentration within immune cells is located either in the blood or the lymph node. G Experimental scheme. Activated T cells were transduced with iGluco probe and re-injected in C57BL/6J mice. Three hours later, activity of iGluco-glucose reporter was analyzed in iGluco-positive cells, isolated from blood and LNs. H Calibration and estimation of glucose concentration was performed by exposing cultured iGluco-expressing T cells to media with different glucose concentrations and by linking iGluco signals to glucose concentration. I iGluco signals in T cells recovered from the blood and LNs of recipient mice. Statistical analyses were performed using a two-sided Wilcoxon test. Compiled from 2 independent experiments with a total of 4 mice. J–L Impact of nutrient availability on T cell energetic charges. CD8 E/EM cells were isolated from blood and LNs of Vav-Cre/Percevalfl/fl mice and exposed to different media composition for 15-30 min. J Experimental scheme. K Heatmaps and L quantification of ATP:ADP ratio and net contributions of OXPHOS, glycolysis, glucose and FA/AA. Statistical analyses were performed using a two-sided one-way ANOVA. *p < 0.05; **p < 0.005; ***p < 0.0005; ****p < 0.0001. Compiled from 7 independent experiments with a total of 7 mice.
Having shown that effector T cells experience distinct glucose levels in blood versus lymph node, we assessed the impact of nutrient availability on T cell energetic charge using defined media compositions (Fig. 4J). Remarkably, blood effector T cells displayed a rapid drop in energetic resources in conditions of reduced glucose availability (glucose-free media, 2DG or Glutor (inhibitor of GLUT1-mediated glucose uptake) treatment) (Fig. 4K, L). This was not the case for effector T cells present in the lymph node. In these cells, we saw a strong upregulation of fatty acid/amino acid usage upon GLUT1 blockade that potentially explains the compensatory increase in ATP:ADP. Conversely, increasing the glucose concentration directly enhanced ATP:ADP levels in blood effector/effector memory CD8+CD44+CD62L- T cells (Supplementary Fig. 3). Effector T cells in lymph nodes exhibit low energetic resources at steady-state and were less sensitive to glucose deprivation (Fig. 4K, L). As expected, naïve T cell ATP:ADP ratio was largely independent on glucose (Supplementary Fig. 4), likely explaining their similar energetic charge in the blood and lymph nodes. Taken together, our data suggest that differential availability of glucose in the blood versus lymph node is the major determinant of differential energetic charges.
Circadian regulation of lymphocyte energetic charges
Immune cell trafficking and functions have been shown to be regulated in a circadian manner18,19,20. We hypothesized that some aspect of the circadian regulation of immunity may be linked to fluctuations in energetic resources. We subjected mice to shifted light regimen for three weeks to be able to simultaneously profile energy metabolism at different zeitgeber time (ZT1 – 1 hour after beginning of the light phase, ZT5, ZT13 – 1 hour after beginning of the dark phase and ZT20) (Fig. 5A). Successful light entrainment was confirmed by observing expected circadian variations of immune cell counts (Supplementary Fig. 5). We next focused on cellular bioenergetics. For CD4+ and CD8+ effector T cells as well as NK and NKT cells, the energetic charge remained higher in the blood as compared to lymph node throughout the day (Fig. 5B, C). Remarkably, lymphocyte energetic resources substantially varied across the day with ATP:ADP ratio changing by two-fold between the minimal and maximal value (Fig. 5B, C). These changes appeared to be of circadian nature as they persisted in constant darkness (Supplementary Fig. 6). For effector cells, change in energetic resources reflected fluctuation in glucose metabolism (Fig. 5B, C) and correlated with diurnal oscillations of glucose concentration in the blood (Supplementary Fig. 7A). Our data suggested a link between oscillations in T cell energetic charge and nutrient intake. To address this point, we compared cellular energetic charge in fed versus fasted mice at ZT1 (corresponding to maximal values of ATP:ADP). As expected, fasted mice displayed lower blood glucose concentrations compared to fed animals (Supplementary Fig. 7B). Importantly, a substantial decrease in energetic charge was observed upon fasting for effector T cells and NK cells while naïve T cells were not affected (Supplementary Fig. 7C). The reduction of ATP:ADP values were also associated with diminished effector T cell size (Supplementary Fig. 7D). Together, our results reveal strong diurnal fluctuations in lymphocyte energetic status, possibly regulated by the availability of glucose or other nutrients.
A–C Vav-Cre/Percevalfl/fl mice were light-entrained for 3 weeks, and later, immune cell populations were isolated from blood and LNs at strict time-points. Later, their energetic resources and metabolic parameters were addressed. A Experimental scheme. Clock image was created in BioRender, and are under license CHIKINA, A. (2025) https://BioRender.com/zx9rjpl. B Heatmaps of ATP:ADP and metabolic parameters of selected immune populations at different time of the day and night. Maximal value − 4.1 (for ATP:ADP), 3.24 (for metabolic parameters); minimal value – 0.46 (for ATP:ADP); 0.11 (for metabolic parameters). C Evolution of ATP:ADP ratio and metabolic parameters throughout the day and night. Mean values ± SEM are shown. Statistical analyses were performed using Kruskal-Wallis-Dunn’s multiple comparisons test. *p < 0.05; **p < 0.005. Statistically different values compared to ZT1 are shown. Compiled from 4 independent experiments with a total of 16 mice.
T cell energetic charge controls effector function
Having shown extensive variations in immune cell energetic resources in time and space, we explored whether these changes would translate into different functional capacities. First, we thought to link the differences in T cell energetic charge in blood versus lymph nodes with their capacity to produce the effector cytokine IFN-γ (Fig. 6A). To this end, activated T cells were adoptively transferred and harvested a day later from the blood and lymph nodes of the recipient. As shown in Fig. 6B T cells recovered from the blood were larger and expressed higher level of IFN-γ and perforin as compared to their lymph node counterparts. A similar hierarchy was observed after in vitro restimulation (Fig. 6C–E).
A–E T cells were isolated from Ubi-GFP OTI Rag-/- mice and activated in vitro. Later, they were adoptively transferred in C57BL/6J mice and harvested a day later from the blood and lymph node of the recipient. A Experimental scheme. Clock image was created in BioRender, and are under license CHIKINA, A. (2025) https://BioRender.com/zx9rjpl. B Production of IFN-γ, Perforin and cell size were addressed by flow cytometry, after a 4 h incubation in the presence of Brefeldin A. RFI, relative fluorescence intensity (MFI for the indicated Ab/MFI of the isotype control). Statistical analyses were performed using a two-sided Wilcoxon test. Production of IFN-γ after re-stimulation with anti-CD3/CD28 (C), N4-peptide (D), and PMA/ionomycin (E). Statistical analyses were performed using a two-sided Wilcoxon test. Compiled from 2 independent experiments with a total of 9 mice. IFN-γ production during different time of the day and night. F Experimental scheme. C57BL/6J mice were light-entrained for 3 weeks, later IFN-γ production by blood CD8E/EM T cells was addressed at specific time-points (G). RFI, relative fluorescence intensity (MFI for IFN-γ/MFI of the isotype control). Mean values ± SEM are shown. Statistical analyses were performed using a Kruskal-Wallis test. Compiled from 3 independent experiments with a total of 24 mice. H, I Manipulation of ATP:ADP ratio by metabolic inhibitors. H Experimental scheme. T cells were isolated from OTI Rag-/- mice and activated for 48 h using an anti-CD3-coated plate in the presence of anti-CD28 and IL-12, transduced or not with Perceval probe, and expanded for 1 week in the presence of Il-2. I T cells were subjected to an array of different Oligomycin and 2DG concentrations and stimulated with N4-peptide for 4 h in the presence of Brefeldin A. Production of IFN-γ and ATP:ADP were analyzed in parallel by flow cytometry, and results were graphed as heatmaps. Association assessed by Pearson’s correlation (two‑sided). Compiled from 4 independent experiments with a total of 6 mice.
Second, we tracked T cell production of IFN-γ throughout the day and night. We revealed that the highest levels of cytokine production were achieved at ZT1, corresponding to the time of maximal energetic charge (Fig. 6G). Of note, changes in IFN-γ production are slightly lagging behind variations of energetic charge, possibly because cytokine levels may result from the integration of cell bioenergetic capacity over the previous hours.
To directly manipulate cellular energetic resources, we altered effector T cell ATP:ADP ratio using multiple combinations of the metabolic inhibitors oligomycin and 2-DG (Fig. 6H, I, Supplementary Fig. 8). For each condition, we recorded the ATP:ADP value and IFN-γ production. Of note, we observed metabolic plasticity in effector T cells as energetic status is primarily affected upon simultaneous inhibition of OXPHOS and glycolysis (Fig. 6I, Supplementary Fig. 8) as previously reported21. Overall, IFN-γ production declined together with decrease in energetic status (Fig. 6I) with a dynamic range of two-fold as also observed in Fig. 6D. A similar pattern was observed when measuring T cell size and intracellular perforin expression as a function of the energetic charge (Supplementary Fig. 9) extending the importance of ATP:ADP ratio to additional hallmarks of T cell activation/function. Of note, reducing glucose concentration by 3-fold, was associated with a trend toward decreased intracellular IFN-γ content in effector CD8+ T cells (Supplementary Fig. 10), suggesting a contribution of glucose availability to T cell energetic charge and effector functions. Together, these experiments established local and temporal variation in energetic charge as a major determinant of T cell function.
Discussion
Previous studies have extensively characterized T cell metabolism in health and disease. Beyond the known regulation of metabolic pathways during T cell responses, we uncovered here important fluctuations in T cell energetic charge during their trafficking to different anatomical sites and at different times of the day that strongly impact their effector functions. We found that effector T cells reached maximal energetic resources once in the blood compared to lymph nodes, due to increased glucose availability. Their energetic charge also varied during the day, reaching maximum values at early rest phase. T cell energetic status had a strong impact on effector functions, establishing ATP:ADP ratio as a novel, dynamic parameter that controls immune responses.
Recent advances have made it possible to study immunometabolism with single-cell resolution. These methods include single-cell RNA sequencing to infer metabolic pathway22, profiling metabolic enzymes and proteins (MET-flow)23, SCENITH that uses protein translation as a readout of ATP levels24, and single-cell metabolite profiling (SpaceM)25. We recently described SPICE-Met to profile energy metabolism by flow cytometry10. Because this method relies on a transgenic mouse expressing a genetically encoded reporter for ATP:ADP ratio, it provides direct and rapid measurement of the cellular energetic charge in defined populations ex vivo. Moreover, when used in combination with metabolic drugs, SPICE-Met can infer the net and relative dependencies on metabolic pathways (OXPHOS vs glycolysis) and nutrients (glucose versus alternative substrate (FA/AA)). With this method, we could profile multiple immune populations simultaneously, revealing strong heterogeneity in energetic status and metabolic parameters.
Interestingly, immune cells displaying high energetic charge showed increased glucose metabolism and a strong net contribution of glycolysis to the cellular ATP:ADP ratio. Immune cells with almost exclusive dependence on OXPHOS, such as naïve and central memory T cells, displayed in comparison lower energetic charges. A striking observation was the drop of energetic charge for several immune cell populations in the lymph node microenvironment. This was specifically seen in effector populations, including CD8+ T cells, NK cells, and NKT cells. Adoptive transfer experiments confirmed the primary role of the organ microenvironment in dictating cell energetic status.
Using an additional reporter for intracellular glucose, we demonstrated that differences in glucose levels between the blood and lymph nodes accounted for the variation of energetic charge in effector cells. Consistently, we observed that immune cells showed evidence of glucose deprivation in the lymph node as reflected by upregulation of GLUT1, a known cellular response to glucose-poor environments14,15,16.
Effector T cells most often develop within lymph nodes before exiting in the circulation and entering peripheral tissue. Our results suggest that the T cells are best equipped with energy resources to fulfill their effector functions after egressing lymph nodes and reaching the circulation. It is tempting to speculate that a lower energetic charge of effector T cells may help control undesirable activities in lymph nodes.
Moreover, effector T cells often egress peripheral tissues and re-enter circulation, raising the interesting possibility that they could regain their energetic resource by benefiting from high glucose availability in the blood. Our results indicated that high glucose availability accounted for the increase in the net contribution of glycolysis and, to a lesser extent, in the net contribution of OXPHOS, together maximizing energy charge.
It is well known that glycolysis is key for T cell effector functions through distinct mechanisms26,27,28,29,30. We found that blocking glucose metabolism only with 2DG had limited impact on ATP:ADP resources but major effects when OXPHOS was also blocked, possibly due to metabolic plasticity. Importantly, depleting energy stores by distinct combinations of metabolic inhibitors strongly reduced T cell function as assessed by production of the effector cytokine IFN-γ, indicating the primary importance of total energetic charge over one specific metabolic pathway, and establishing a causal relationship between effector T cell energetic resources and function. In line with these results, adoptively transferred effector T cells showed increased size, IFN-γ production, and perforin content when reaching the blood, where the energetic status is maximal as compared to lymph nodes.
In an elegant study, Pearce and colleagues demonstrated that transient glucose restriction in effector T cells in vitro conditioned them for enhanced activity upon glucose re-exposure, in part due to GLUT1 upregulation31. In this respect, it is interesting to consider that the glucose-poor lymph node microenvironment may represent a place of transient glucose restriction that further boosts T cell activity upon re-exposure to blood glucose levels. It will be interesting to further consider the existence of nutrient niches in the lymph node impacting the T cell activation process.
In addition to spatial fluctuation, we observed circadian variations in lymphocyte energetic charge that were maximal at early rest-phase. These variations were largely due to increased net contribution of glycolysis and glucose metabolism. Blood glucose levels are known to oscillate during the day in human32 and mice33,34,35, in part due to the feeding regimen. Interestingly, the peak of glucose appears to overlap with the time of maximal energy resource and glucose metabolism observed in our study. Focusing on effector T cells, we again observed that cytokine production reached its highest level when energetic resources were maximal (early rest-phase).
Several studies have highlighted circadian regulation of immune cell function18,19,20. We propose that fluctuations in energetic charge may represent a mechanistic intermediate for some of these differences.
In sum, we propose that, in addition to transcriptional, epigenetic, and metabolic changes, spatiotemporal variations of T cell energetic charge due to dynamic nutrient availability represent a key parameter for the control of immune effector functions.
Materials and Methods
Mice
C57BL/6 J mice, aged 6 to 8 weeks, were purchased from ENVIGO and housed in the animal care facility at Institut Pasteur under specific pathogen-free conditions. Vav-iCre x Percevalfl/fl (SPICY), GFP-OT-I-Rag1-/-, CD45.1 mice were bred on-site at our facility. Experimental/control animals were co-housed. Both male and female mice were used, except for circadian experiments, which were conducted on males. Mice have been euthanized using a CO2 chamber in agreement with ethical requirements. All animal experiments were conducted with approval from the Institut Pasteur Safety Committee, in compliance with French and European guidelines (CETEA 230038) and were validated by the French Ministry of Education and Research.
Sample preparation
Blood was collected by 0.5 G insulin syringes containing 50 µL of 2 mM EDTA by cardiac puncture. 1 mL of blood sample was processed using RBC lysis buffer (eBioscience), following manufacturer's instructions. Lymph nodes were crushed on 70 µm cell strainers (Corning) with Injekt Luer Solo 2 mL syringes plug, lymphocytes have been collected in R-10 complete media (RPMI 1640 Glutamax without phenol-red; 10% heat-inactivated FCS,; 50 µ/ml Penicillin-streptomycin; 10 mM HEPES, 50 µM b-mercaptoethanol, Gibco) and centrifuged. After the last centrifugation, blood and lymph node cells were resuspended in R-10 complete media for further staining.
SPICE-Met Flow cytometry
Cells expressing PercevalHR were analyzed by flow cytometry. ATP:ADP ratios (R) in individual cells were calculated from two fluorescence signals. In brief, excitation with a violet laser (405 nm) and signal detection with a 525 band-pass filter were used to measure ADP contribution, while ATP levels were estimated by excitation with a blue laser (488 nm) and signal detection with a 525 band-pass filter. To calculate ATP:ADP ratio, we derived a new parameter (FlowJo) corresponding to fluorescence signals collected in the ATP channel divided by the fluorescent signals from the ADP channel. To reveal OXPHOS/glycolysis contribution, cells have been treated for 10 min at 37 °C with 1 µM oligomycin (oligo, Sigma); to reveal glucose/fatty and amino acids contribution, cells have been treated with 1 mM iodoacetic acid (IA, Sigma) or 100 mM 2-deoxy-D-glucose (2DG, Sigma). A combination of oligomycin with iodoacetic acid treatment was used to reveal the minimal level of energetic resources. Control samples were treated with H2O and DMSO. Relative OXPHOS/glycolysis dependencies were calculated as ((RDMSO+H2O - ROligomycin)/(RDMSO+H2O – ROligo+IA)) × 100 and ((ROligomycin – ROligo+IA)/(RDMSO+H2O – ROligo+IA)), respectively. Absolute OXPHOS/glycolysis dependencies were calculated as (RDMSO+H2O - ROligomycin) and (ROligomycin – Roligo+IA), respectively. Relative glucose/fatty and amino acids dependencies were calculated as ((RDMSO+H2O – RIA)/(RDMSO+H2O – ROligo+IA)) × 100 and ((RIA – ROligo+IA)/(RDMSO+H2O – ROligo+IA)), respectively. Absolute glucose/fatty and amino acids dependencies were calculated as (RDMSO+H2O – RIA) and (RIA – Roligo+IA), respectively.
MVA vaccination
The recombinant MVA-HIV-B, which encodes full-length HIV Gag along with three Pol and two Nef fragments12 was supplied by the Agence Nationale de Recherche sur le Sida (ANRS). Mice were injected with 2 × 10⁶ p.f.u. of MVA-HIV-B via footpad injection.
Adoptive transfer
Lymphocytes were isolated from lymph nodes of Vav-iCre x Percevalfl/fl (SPICY) mice (expressing CD45.2 congenic marker), and the resulting cell suspension was injected intravenously (2 × 107 cells per mouse) in CD45.1 animals of the same sex. Recipients were killed 3 h later, and lymphocytes isolated from blood and lymph nodes were processed for SPICE-Met profiling.
Generation of T cells expressing the glucose reporter iGluco
The construct of encoding the iGlucoSnFR-mRuby2 (iGluco) glucose probe17 was obtained from Addgene and subcloned into the retroviral vector MSCV. T cells were activated in plates coated with anti-CD3 monoclonal antibody (mAb) (2.5 μg ml−1; clone 17.A2; BioLegend) in the presence of soluble anti-CD28 mAb (2.5 μg ml−1; clone 37.51; BioLegend) and murine IL-12 (10 ng ml−1; I8523; Sigma-Aldrich)36. Two rounds of spin infection were performed at 24 and 48 hours after T cell activation using retroviral particles supplemented with polybrene (8 μg ml−1; Merck). T cells were cultured for four additional days in the presence of hIL-2 (10 ng ml−1; 202-IL; R&D Systems). Calibration of the iGluco probe was performed in R10 glucose-free media, supplemented with different glucose concentrations (D-Glucose, Sigma).
Impact of nutrient availability
Lymphocytes, isolated from Vav-iCre/Percevalfl/fl (SPICY) mice, were isolated and stained as described above. Later, they were subjected to different media for at least 20 min at 37 °C 5% CO2 incubator, and their ATP: ADP ratio was analyzed. Media were prepared based on the SILAC RPMI 1640 Flex Media without glucose (Gibco), with the following additives, according to experimental condition: D-glucose (Gibco), fatty acids supplement (1:100, Sigma), GlutaMAX (1:100, Gibco), Glutor (4 µM, Sigma), 2DG (100 mM, Sigma). HEPES, penicillin, streptomycin and b-mercaptoethanol were added in all conditions, as described above for complete R-10 media composition. The addition of metabolic inhibitors and the reading of ATP:ADP ratio were performed directly in the experimental media.
Circadian experiments
In all cases, Vav-iCre/Percevalfl/fl (SPICY) and C57BL/6J male mice were entrained to a 12-h light:12-h dark cycle for at least 3 weeks before experimentation, using Leddy programmable IVC lighting control system (Techniplast). Zeitgeber time (ZT) 0 was defined as the time of lights on, and ZT12 is the time of lights off.
Generation of effector OT-I CD8+ T cells
In vitro OT-I T cell stimulation was performed during 4 h in complete R10 media, supplemented with 0.1% brefeldin (BD GolgiPlug, BD Biosciences) and, according to experimental conditions, supplemented with phormol 12-myristate 13-acetate (0.02 µg/mL PMA, Sigma) and ionomycin calcium salt from Streptomyces conglobatus (0.5 µg/mL, Sigma); or plated on anti-CD3 antibody coated plates (2.5 µg/mL, clone 17A2, Biolegend) and, in this case, supplemented with anti-CD28 (2.5 µg/mL, clone 37.51, Biolegend). In case of N4-peptide re-stimulation, SIINFEKL peptide (10 µM, AS-60193-5, Anaspec) was used.
Flow cytometry
For standard cell surface staining, the cells were incubated in R-10 complete media. All antibodies were purchased from Biolegend, if not noted otherwise. For extracellular staining cells have been incubated for 20 min at 4 °C with constant agitation with CD8-BUV395 (1:200, clone 53-6.7, BD Biosciences), CD11b-BUV736 (1:200, clone M1/70, BD Biosciences), CD44-BV605 (1:200, clone IM7), CD4-BV786 (1:200, clone RM4-5), NK1.1-BV650 (1:200, clone PK136), TCRβ-APCCy7 (1:200, clone H57-597), Ly6G-Alexa647 (1:200, clone 1A8), CD19-PECy7 (1:200, clone 6D5), CD62L-BV421 (1:200, clone MEL-14), CD45.1-PECy7 (1:50, clone A20), CD45.2-BUV736 (1:50, clone 104, BD Biosciences), GLUT1 (1:50, Sigma 07-1401-AF647 or Almone labs AGT-041 or Biotechne clone 202915), H2-Kb-TSYKFESV MHC:peptide tetramers were generously provided by the National Institutes of Health Tetramer Core Facility. Tetramer staining was conducted first, followed by staining with the antibody cocktail. For intracellular staining, cells have been processed with BD Cytofix/Cytoperm™ Fixation/Permeabilization Kit, according to manufacturer instructions. Incubations with anti-IFN-γ (1:50, clone XMG1.2) and anti-Perforin (1:50, clone S16009B) antibodies were done overnight at +4 °C with constant agitation. Isotype controls were PE-Rat IgG1, κ; clone RTK2071 (for IFN-γ staining) and APC-Rat IgG2a, κ; clone RTK2758 (for perforin staining). Dead cells were stained and excluded by fixable viability dye eF780 (1:1000, Invitrogen), added to extracellular antibodies cocktail mix. Data were collected using a Cytoflex LX flow cytometer (Beckman Culture) and analyzed using FlowJo software v10.8.1 (BD Biosciences).
Statistical analysis
All statistical analyses were conducted using Prism v.9.5.0 (GraphPad). Data are presented as mean ± SEM. The Wilcoxon signed-rank test was used to compare paired groups. The Mann-Whitney U test was used for comparing two independent groups or a paired t-test if normality was validated by a Shapiro-Wilk test. For comparison of multiple (>2) independent groups, we used Kruskal-Wallis-Dunn’s test or one-way ANOVA if normality was validated by a Shapiro-Wilk test. All statistical tests were two-tailed with a significance level of 0.05. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001; ns, non-significant.
Graphical design
Figures 1 A, 2A, D, 3A, 4A, D, G, J, 5A, 6A, F, H, supplementary Fig. 6A, were created in-house using Adobe Illustrator software. Clock images in Figs. 5A, 6F, supplementary Fig. 6A, were created in BioRender, and are under license CHIKINA, A. (2025) https://BioRender.com/zx9rjpl.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
All data generated in this study are available within the article, its Supplementary Information, and the associated Source Data files. Source data are provided with this paper.
References
Chapman, N. M., Boothby, M. R. & Chi, H. Metabolic coordination of T cell quiescence and activation. Nat. Rev. Immunol. 20, 55–70 (2020).
Geltink, R. I. K., Kyle, R. L. & Pearce, E. L. Unraveling the complex interplay between T Cell metabolism and function. Annu. Rev. Immunol. 36, 461–488 (2018).
B. Alberts, et al, Catalysis and the Use of Energy by Cells. Molecular Biology of the Cell, 4th edition (2002).
Atkinson, D. E. The energy charge of the adenylate pool as a regulatory parameter. Interaction with feedback modifiers. Biochemistry 7, 4030–4034 (1968).
De la Fuente, I. M. et al. On the dynamics of the adenylate energy system: homeorhesis vs homeostasis. PLoS One 9, e108676 (2014).
Berg, J., Hung, Y. P. & Yellen, G. A genetically encoded fluorescent reporter of ATP:ADP ratio. Nat. Methods 6, 161–166 (2009).
von Andrian, U. H. & Mempel, T. R. Homing and cellular traffic in lymph nodes. Nat. Rev. Immunol. 3, 867–878 (2003).
Abbondante, S., Eckel-Mahan, K. L., Ceglia, N. J., Baldi, P. & Sassone-Corsi, P. Comparative Circadian metabolomics reveal differential effects of nutritional challenge in the serum and liver. J. Biol. Chem. 291, 2812–2828 (2016).
Cosgrove, J. et al. A call for accessible tools to unlock single-cell immunometabolism research. Nat. Metab. 6, 779–782 (2024).
Russo, E. et al. SPICE-Met: profiling and imaging energy metabolism at the single-cell level using a fluorescent reporter mouse. EMBO J. 41, e111528 (2022).
Tantama, M., Martinez-Francois, J. R., Mongeon, R. & Yellen, G. Imaging energy status in live cells with a fluorescent biosensor of the intracellular ATP-to-ADP ratio. Nat. Commun. 4, 2550 (2013).
Sagoo, P. et al. In vivo imaging of inflammasome activation reveals a subcapsular macrophage burst response that mobilizes innate and adaptive immunity. Nat. Med. 22, 64–71 (2016).
Macintyre, A. N. et al. The glucose transporter Glut1 is selectively essential for CD4 T cell activation and effector function. Cell Metab. 20, 61–72 (2014).
Qualls-Histed, S. J., Nielsen, C. P. & MacGurn, J. A. Lysosomal trafficking of the glucose transporter GLUT1 requires sequential regulation by TXNIP and ubiquitin. iScience 26, 106150 (2023).
Kumagai, A. K., Kang, Y. S., Boado, R. J. & Pardridge, W. M. Upregulation of blood-brain barrier GLUT1 glucose transporter protein and mRNA in experimental chronic hypoglycemia. Diabetes 44, 1399–1404 (1995).
Smoak, I. W. & Branch, S. Glut-1 expression and its response to hypoglycemia in the embryonic mouse heart. Anat. Embryol. 201, 327–333 (2000).
Keller, J. P. et al. In vivo glucose imaging in multiple model organisms with an engineered single-wavelength sensor. Cell Rep. 35, 109284 (2021).
Fortier, E. E. et al. Circadian variation of the response of T cells to antigen. J. Immunol. 187, 6291–6300 (2011).
Druzd, D. et al. Lymphocyte Circadian clocks control lymph node trafficking and adaptive immune responses. Immunity 46, 120–132 (2017).
Nobis, C. C. et al. The circadian clock of CD8 T cells modulates their early response to vaccination and the rhythmicity of related signaling pathways. Proc. Natl. Acad. Sci. USA 116, 20077–20086 (2019).
Russo, E. et al. SPICE-Met: profiling and imaging energy metabolism at the single-cell level using a fluorescent reporter mouse. EMBO J, e111528 (2022).
Hartmann, F. J. et al. Single-cell metabolic profiling of human cytotoxic T cells. Nat. Biotechnol. 39, 186–197 (2021).
Ahl, P. J. et al. Met-Flow, a strategy for single-cell metabolic analysis highlights dynamic changes in immune subpopulations. Commun. Biol. 3, 305 (2020).
Arguello, R. J. et al. SCENITH: A flow cytometry-based method to functionally profile energy metabolism with single-cell resolution. Cell Metab. 32, 1063–1075 e1067 (2020).
Rappez, L. et al. SpaceM reveals metabolic states of single cells. Nat. Methods 18, 799–805 (2021).
Ho, P. C. et al. Phosphoenolpyruvate is a metabolic checkpoint of anti-tumor T cell responses. Cell 162, 1217–1228 (2015).
Chang, C. H. et al. Posttranscriptional control of T cell effector function by aerobic glycolysis. Cell 153, 1239–1251 (2013).
Cham, C. M., Driessens, G., O’Keefe, J. P. & Gajewski, T. F. Glucose deprivation inhibits multiple key gene expression events and effector functions in CD8+ T cells. Eur. J. Immunol. 38, 2438–2450 (2008).
Finlay, D. K. et al. PDK1 regulation of mTOR and hypoxia-inducible factor 1 integrate metabolism and migration of CD8+ T cells. J. Exp. Med. 209, 2441–2453 (2012).
Michalek, R. D. et al. Cutting edge: distinct glycolytic and lipid oxidative metabolic programs are essential for effector and regulatory CD4+ T cell subsets. J. Immunol. 186, 3299–3303 (2011).
Klein Geltink, R. I. et al. Metabolic conditioning of CD8(+) effector T cells for adoptive cell therapy. Nat. Metab. 2, 703–716 (2020).
Scheen, A. J., Byrne, M. M., Plat, L., Leproult, R. & Van Cauter, E. Relationships between sleep quality and glucose regulation in normal humans. Am. J. Physiol. 271, E261–E270 (1996).
Ando, H., Ushijima, K., Shimba, S. & Fujimura, A. Daily fasting blood glucose rhythm in male mice: a role of the Circadian clock in the liver. Endocrinology 157, 463–469 (2016).
Owino, S., Contreras-Alcantara, S., Baba, K. & Tosini, G. Melatonin signaling controls the daily rhythm in blood glucose levels independent of peripheral clocks. PLoS One 11, e0148214 (2016).
Zhong, L. X. et al. Circadian misalignment alters insulin sensitivity during the light phase and shifts glucose tolerance rhythms in female mice. PLoS One 14, e0225813 (2019).
Andrijauskaite, K. et al. IL-12 conditioning improves retrovirally mediated transduction efficiency of CD8+ T cells. Cancer Gene Ther. 22, 360–367 (2015).
Acknowledgements
We are grateful to the members of the Bousso laboratory for their valuable feedback on the manuscript. We acknowledge the mouse facility at Institut Pasteur for their support in conducting this study. We also thank the NIH Tetramer Core Facility for supplying MHC-peptide tetramers. We thank R. Alonso for help with mouse experiments and C.L. Grandjean for ethic protocols. The work was supported by Institut Pasteur, INSERM, an Advanced grant (ENLIGHTEN) from the European Research Council (P.B), an ANR Grant (Metaniche), and by the Investissements d’Avenir program managed by the ANR (ANR-10-LABX-77-01). ASC. was supported by Fondation pour la Recherche Médicale (SPF202005012006).
Author information
Authors and Affiliations
Contributions
A.S.C., B.C., and F.L. conducted the experiments, A.S.C. and P.B. analyzed the data, A.S.C. and P.B. designed the experiments, and A.S.C. and P.B. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Chikina, A.S., Corre, B., Lemaître, F. et al. Spatiotemporal regulation of energetic charge dictates T cell function. Nat Commun 17, 770 (2026). https://doi.org/10.1038/s41467-026-68559-1
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41467-026-68559-1