Abstract
Bystander editing remains a major limitation of current base editors, hindering their precision and therapeutic potential. Here, we present a de novo protein design strategy that creates a structurally rigid interface between a DNA-binding TALE domain and a cytosine deaminase, forming a unified editing module termed TALE-oriented deaminase (TOD). Cryo-EM analysis of TOD–DNA complexes confirms that this precise spatial architecture tightly restricts the deaminase activity window, thereby minimizing unwanted deamination. To further enhance editing specificity, we develop a split version, termed DdCBE–TOD, which virtually eliminates off-target editing. As a proof of concept, we apply DdCBE–TOD to generate a mitochondrial disease mouse model and to correct a pathogenic mutation associated with MERRF syndrome in patient-derived cells, achieving single-nucleotide precision. This work introduces a generalizable and computationally guided approach for ultra-precise base editing, offering a promising platform for both mechanistic studies and therapeutic correction of single-nucleotide mutations.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
Raw data of whole mitochondrial genome sequencing for off-target analysis are available as a BioProject with the project identifier PRJCA038551 (https://ngdc.cncb.ac.cn/gsa-human/s/88Zs4Xcl) in the China National Center for Bioinformation–National Genomics Data Center database55,56. Cryo-EM density maps and structure coordinates have been deposited in the Electron Microscopy Data Bank and the Protein Data Bank, with accession codes EMD-62996 and PDB 9LCY for TOD6inact with the TC dsDNA substrate; EMD-62999 and PDB 9LD1 for TOD4inact with the TC dsDNA substrate, along with a local map of the orienting domain and the deaminase region; EMD-62997 and PDB 9LCZ for TOD6inact with the GC dsDNA substrate; EMD-62995 and PDB 9LCX for TOD6inact with the AC dsDNA substrate; and EMD-62998 and PDB 9LD0 for TOD6inact with the CC dsDNA substrate, and corresponding cryo-EM maps, half-maps and models are also provided through Figshare (https://figshare.com/s/2f675005689b82275513)57. Source data are provided with this paper.
References
Silva-Pinheiro, P. & Minczuk, M. The potential of mitochondrial genome engineering. Nat. Rev. Genet. 23, 199–214 (2022).
Bacman, S. R., Williams, S. L., Pinto, M., Peralta, S. & Moraes, C. T. Specific elimination of mutant mitochondrial genomes in patient-derived cells by mitoTALENs. Nat. Med. 19, 1111–1113 (2013).
Gammage, P. A., Rorbach, J., Vincent, A. I., Rebar, E. J. & Minczuk, M. Mitochondrially targeted ZFNs for selective degradation of pathogenic mitochondrial genomes bearing large-scale deletions or point mutations. EMBO Mol. Med. 6, 458–466 (2014).
Mok, B. Y. et al. A bacterial cytidine deaminase toxin enables CRISPR-free mitochondrial base editing. Nature 583, 631–637 (2020).
Cho, S.-I. et al. Targeted A-to-G base editing in human mitochondrial DNA with programmable deaminases. Cell 185, 1764–1776 (2022).
Yi, Z. et al. Strand-selective base editing of human mitochondrial DNA using mitoBEs. Nat. Biotechnol. 42, 498–509 (2024).
Hu, J. et al. Strand-preferred base editing of organellar and nuclear genomes using CyDENT. Nat. Biotechnol. 42, 936–945 (2024).
Lim, K., Cho, S.-I. & Kim, J.-S. Nuclear and mitochondrial DNA editing in human cells with zinc finger deaminases. Nat. Commun. 13, 366 (2022).
Willis, J. C. W., Silva-Pinheiro, P., Widdup, L., Minczuk, M. & Liu, D. R. Compact zinc finger base editors that edit mitochondrial or nuclear DNA in vitro and in vivo. Nat. Commun. 13, 7204 (2022).
Zhang, X., et al. Precise modelling of mitochondrial diseases using optimized mitoBEs. Nature 639, 735–745 (2025).
Chu, A. E., Lu, T. & Huang, P.-S. Sparks of function by de novo protein design. Nat. Biotechnol. 42, 203–215 (2024).
Listov, D., Goverde, C. A., Correia, B. E. & Fleishman, S. J. Opportunities and challenges in design and optimization of protein function. Nat. Rev. Mol. Cell Biol. 25, 639–653 (2024).
Kortemme, T. De novo protein design—from new structures to programmable functions. Cell 187, 526–544 (2024).
Deng, D. et al. Structural basis for sequence-specific recognition of DNA by TAL effectors. Science 335, 720–723 (2012).
Mi, L. et al. DddA homolog search and engineering expand sequence compatibility of mitochondrial base editing. Nat. Commun. 14, 874 (2023).
Yin, L., Shi, K. & Aihara, H. Structural basis of sequence-specific cytosine deamination by double-stranded DNA deaminase toxin DddA. Nat. Struct. Mol. Biol. 30, 1153–1159 (2023).
Mok, Y. G. et al. Base editing in human cells with monomeric DddA–TALE fusion deaminases. Nat. Commun. 13, 4038 (2022).
Watson, J. L. et al. De novo design of protein structure and function with RFdiffusion. Nature 620, 1089–1100 (2023).
Dauparas, J. et al. Robust deep learning-based protein sequence design using ProteinMPNN. Science 378, 49–56 (2022).
Jumper, J. et al. Highly accurate protein structure prediction with AlphaFold. Nature 596, 583–589 (2021).
Mok, B. Y. et al. CRISPR-free base editors with enhanced activity and expanded targeting scope in mitochondrial and nuclear DNA. Nat. Biotechnol. 40, 1378–1387 (2022).
Neugebauer, M. E. et al. Evolution of an adenine base editor into a small, efficient cytosine base editor with low off-target activity. Nat. Biotechnol. 41, 673–685 (2023).
Doyle, E. L. et al. TAL Effector–Nucleotide Targeter (TALE-NT) 2.0: tools for TAL effector design and target prediction. Nucleic Acids Res. 40, W117–W122 (2012).
Hameed, S. & Tadi, P. Myoclonic Epilepsy and Ragged Red Fibers (StatPearls, 2023).
Ripolone, M., et al. MERRF mutation A8344G in a four-generation family without central nervous system involvement: clinical and molecular characterization. J. Pers. Med. 13, 147 (2023).
Burrage, L. C. et al. Mitochondrial myopathy, lactic acidosis, and sideroblastic anemia (MLASA) plus associated with a novel de novo mutation (m.8969G>A) in the mitochondrial encoded ATP6 gene. Mol. Genet. Metab. 113, 207–212 (2014).
Wen, S. et al. Identification of G8969>A in mitochondrial ATP6 gene that severely compromises ATP synthase function in a patient with IgA nephropathy. Sci. Rep. 6, 36313 (2016).
Lee, S., Lee, H., Baek, G. & Kim, J.-S. Precision mitochondrial DNA editing with high-fidelity DddA-derived base editors. Nat. Biotechnol. 41, 378–386 (2023).
Lei, Z. et al. Mitochondrial base editor induces substantial nuclear off-target mutations. Nature 606, 804–811 (2022).
Kauppila, J. H. K. et al. A phenotype-driven approach to generate mouse models with pathogenic mtDNA mutations causing mitochondrial disease. Cell Rep. 16, 2980–2990 (2016).
Zhang, L. et al. Age-dependent accumulation of mitochondrial tRNA mutations in mouse kidneys linked to mitochondrial kidney diseases. Nat. Aging 5, 1317–1339 (2025).
Lott, M. T. et al. mtDNA variation and analysis using Mitomap and Mitomaster. Curr. Protoc. Bioinformatics 44, 1.23.21–21.23.26 (2013).
Nguyen, E. et al. Sequence modeling and design from molecular to genome scale with Evo. Science 386, eado9336 (2024).
He, Y. et al. Protein language models-assisted optimization of a uracil-N-glycosylase variant enables programmable T-to-G and T-to-C base editing. Mol. Cell 84, 1257–1270 (2024).
Guo, J. et al. A DddA ortholog-based and transactivator-assisted nuclear and mitochondrial cytosine base editors with expanded target compatibility. Mol. Cell 83, 1710–1724 (2023).
Huang, J. et al. Discovery of deaminase functions by structure-based protein clustering. Cell 186, 3182–3195 (2023).
Sun, H. et al. Developing mitochondrial base editors with diverse context compatibility and high fidelity via saturated spacer library. Nat. Commun. 14, 6625 (2023).
Cheng, K. Engineering RsDddA as mitochondrial base editor with wide target compatibility and enhanced activity. Mol. Ther. Nucleic Acids 34, 102028 (2023).
Fauser, F. et al. Compact zinc finger architecture utilizing toxin-derived cytidine deaminases for highly efficient base editing in human cells. Nat. Commun. 15, 1181 (2024).
Cho, S.-I. et al. Engineering TALE-linked deaminases to facilitate precision adenine base editing in mitochondrial DNA. Cell 187, 95–109 (2024).
Kang, B.-C. et al. Chloroplast and mitochondrial DNA editing in plants. Nat. Plants 7, 899–905 (2021).
Kim, J.-S. & Chen, J. Base editing of organellar DNA with programmable deaminases. Nat. Rev. Mol. Cell Biol. 25, 34–45 (2024).
Mukherjee, S. & Zhang, Y. MM-align: a quick algorithm for aligning multiple-chain protein complex structures using iterative dynamic programming. Nucleic Acids Res. 37, e83 (2009).
Yang, J., Guo, S., Yuan, P. & Wei, W. in TALENs: Methods and Protocols (eds Kühn, R. et al.) 49–60 (Springer, 2016).
Thompson, R. F., Iadanza, M. G., Hesketh, E. L., Rawson, S. & Ranson, N. A. Collection, pre-processing and on-the-fly analysis of data for high-resolution, single-particle cryo-electron microscopy. Nat. Protoc. 14, 100–118 (2019).
Punjani, A., Rubinstein, J. L., Fleet, D. J. & Brubaker, M. A. cryoSPARC: algorithms for rapid unsupervised cryo-EM structure determination. Nat. Methods 14, 290–296 (2017).
Zheng, S. Q. et al. MotionCor2: anisotropic correction of beam-induced motion for improved cryo-electron microscopy. Nat. Methods 14, 331–332 (2017).
Xu, K., Wang, Z., Shi, J., Li, H. & Zhang, Q. C. A2-Net: molecular structure estimation from cryo-EM density volumes. In Proc. AAAI Conference on Artificial Intelligence Vol. 33, 1230–1237 (AAAI, 2019).
Emsley, P. & Cowtan, K. Coot: model-building tools for molecular graphics. Acta Crystallogr. D Biol. Crystallogr. 60, 2126–2132 (2004).
Song, Y. et al. High-resolution comparative modeling with RosettaCM. Structure 21, 1735–1742 (2013).
Pettersen, E. F. et al. UCSF ChimeraX: structure visualization for researchers, educators, and developers. Protein Sci. 30, 70–82 (2021).
Clement, K. et al. CRISPResso2 provides accurate and rapid genome editing sequence analysis. Nat. Biotechnol. 37, 224–226 (2019).
Diroma, M. A., Ciaccia, L., Pesole, G. & Picardi, E. Elucidating the editome: bioinformatics approaches for RNA editing detection. Brief. Bioinform. 20, 436–447 (2017).
Ru, Y. et al. Maternal age enhances purifying selection on pathogenic mutations in complex I genes of mammalian mtDNA. Nat. Aging 4, 1211–1230 (2024).
Chen, T. et al. The Genome Sequence Archive family: toward explosive data growth and diverse data types. Genomics Proteomics Bioinformatics 19, 578–583 (2021).
CNCB-NGDC Members and Partners. Database resources of the National Genomics Data Center, China National Center for Bioinformation in 2022. Nucleic Acids Res. 50, D27–D38 (2021).
Computational design of a high-precision mitochondrial DNA cytosine base editor. Figshare https://figshare.com/s/2f675005689b82275513 (2025).
Acknowledgements
We thank the staff of cryo-EM facility and of the HPC Center of Westlake University for their support. We thank W. Wei and Y. Yu for providing templates for assembling TALE protein. This work was funded by the National Key Research and Development Program of China (2024YFC3405500 to L.M. and 2021YFA1100200 to Y.W.), the National Natural Science Foundation of China (32525039, 32430063 and 22137005 to P.L.; 32025007 and 32130017 to Y.W.; 82271897 to M.J.), the State Key Laboratory of Gene Expression (grant no. 2025ZY01117), the Zhejiang Provincial Natural Science Foundation of China (LR23C050001 to P.L. and LZYQ25C070001 to M.J.), the ‘Pioneer’ and ‘Leading Goose’ R&D Program of Zhejiang (grant no. 2024SSYS0036), the China Postdoctoral Science Foundation (2024M762947 to L.M.) and the Westlake Center for Genome Editing of Westlake University, program no. 21200000A992410/003. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
L.M., Y.W. and P.L. conceived the research and designed experiments. L.M. designed the orienting domains. L.M., Y.-X.L. and Z.-L.W. completed molecular cloning. L.M. performed the E. coli-based editing assay and protein purification. X. Lv performed the in vitro deamination assay. H.L. prepared cryo-EM samples. X. Lv and L.M. collected cryo-EM data and solved the EM structures. Y.-X.L. performed the mammalian cell base-editing assay with assistance from Z.-L.W. Y.-X.L. constructed off-target editing libraries. L.M. and Y.-X.L. analyzed the sequencing data. Y.Y. provided assistance in experiments. X. Liu, L.Z., Z.X. and M.J. characterized the application of DdCBE–TODs in MEF cells and mice. X.Z. and K.J. provided primary skin fibroblasts with the m.A8344G mutation. K.Z. carried out molecular dynamics simulations. P.L., Y.W. and L.M. wrote the manuscript with X. Lv’s and Y.-X.L.’s assistance.
Corresponding authors
Ethics declarations
Competing interests
L.M. and P.L. are inventors on a provisional patent application submitted by Westlake University; the remaining authors declare no competing interests.
Peer review
Peer review information
Nature Structural & Molecular Biology thanks Sangsu Bae, Kozo Tomita, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editors: Sara Osman and Melina Casadio, in collaboration with the Nature Structural & Molecular Biology team. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 mDdCBEs using a truncated Ddd_Ss variant demonstrate significant mtDNA editing activity.
a, Architectures of DdCBE and mDdCBEs for mtDNA editing. A truncated Ddd_Ss variant (Ddd_Ss_S, denoting Ddd_Ss_Short, residues 2–114), which lacks the C-terminal SPKK-related motif, was utilized to construct mDdCBEs. MTS, mitochondrial localization sequences. b, Heat maps showing C•G-to-T•A conversion frequencies for DdCBE and mDdCBEs at the MT-ND6 site in mtDNA of HEK293T cells. Shown are individual values from n = 3 independent experiments. c, Indel frequencies of DdCBE and mDdCBEs at the MT-ND6 site in mtDNA. Shown are mean ± SD from n = 3 independent experiments. The transfection duration was 3 days. Source data are provided as a Source Data file.
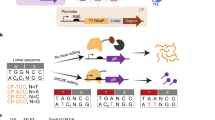
Extended Data Fig. 2 Base editing by mDdCBE-TODs in E. coli-based editing assays.
a, b, The editing efficiency of mDdCBE and five designer mDdCBE-TODs at various positions of the four DNA substrates (as shown in Fig. 1c). The numbers on the X-axis represent the distance, measured in nucleotides, to the last nucleotide in the TALE binding sequence. mDdCBE and mDdCBE-TODs were induced with 0.02 g/L (a) or 0.2 g/L (b) arabinose for 1 h. Data are presented as mean ± SD from n = 3 independent experiments. Source data are provided as a Source Data file.
Extended Data Fig. 3 In vitro cytosine deamination assays for mDdCBE and TOD6.
a, In vitro cytosine deamination activity of mDdCBE and TOD6. dsDNA substrates (S1–S4) were labeled with 6-Carboxyfluorescein (FAM, shown as a green star). The DNA sequence is presented at the top, with cytosines highlighted in red. The white bar denotes the TALE binding sequence, and the purple bar denotes the complementary sequence. Cytosine deamination results in cleavage by further treatment (Methods), generating cleaved products (P) with increased mobility. b, In vitro cytosine deamination activity of TOD6 against four dsDNA substrates (S) with 5′-GC, AC, TC, or CC target sequences. Representative gel images from n = 3 independent experiments are shown. Source data are provided as a Source Data file.
Extended Data Fig. 4 Cryo-EM structure of TOD4 complexed with dsDNA substrate.
a, Cryo-EM structure of TOD4inact complexed with dsDNA substrate (colored) is in close agreement with the design model (gray). Schematic diagram (top) shows the domain organization of TOD4, comprising TALE, orienting domain C4 (candidate 4), and Ddd_Ss_S deaminase. b, The zoomed view showing the last TALE repeat (purple), orienting domain C4 (blue) and inactive Ddd_Ss_S (pink) in TOD4 interacting with dsDNA substrate (orange). c, d, Detailed views showing that residues from the orienting domain C4 form extensive interactions with both the last TALE repeat and the Ddd_Ss_S deaminase. e, Cryo-EM structures of Ddd_Ss_S in TOD6inact and TOD4inact complexed with dsDNA substrate are almost identical.
Extended Data Fig. 5 mtDNA editing by mDdCBE-TOD6 in HEK293T cells.
a, Architectures of mDdCBE and mDdCBE-TOD6 for mtDNA editing. b–f, mtDNA editing by mDdCBE (pink) and mDdCBE-TOD6 (blue) at the MT-CYB (b), MT-ND1.1 (c), MT-ND3 (d), MT-ND4 (e) and MT-TK.1 (f) sites. In panel (c), a putative TALE binding site that is highly similar to the designated TALE binding site is indicated. Sequencing data from untreated cells (grey) are shown as control. Data are presented as mean ± SD from n = 3 independent experiments. The transfection duration was 3 days. Source data are provided as a Source Data file.
Extended Data Fig. 6 On-target and off-target editing activities of mDdCBE-TOD6.
a–f, Average C•G to T•A editing efficiencies at on-target (red dots, designated target site) and off-target (gray dots) sites across mtDNA for MT-CYB-mDdCBE (a), MT-CYB-mDdCBE-TOD6 (b), MT-ND1.1-mDdCBE (c), MT-ND1.1-mDdCBE-TOD6 (d), MT-ND3-mDdCBE (e) and MT-ND3-mDdCBE-TOD6 (f). Sites with average editing frequency greater than 1% are shown. Data are shown as means from n = 2 independent experiments. g, Venn diagram showing the overlap of off-target sites among MT-CYB-mDdCBE, MT-ND1.1-mDdCBE and MT-ND3-mDdCBE. h, Venn diagram showing the overlap of off-target sites among MT-CYB-mDdCBE-TOD6, MT-ND1.1-mDdCBE-TOD6 and MT-ND3-mDdCBE-TOD6. Source data are provided as a Source Data file.
Extended Data Fig. 7 Splitting at N67 (DdCBE-TOD6_N67) achieves efficient and precise editing.
a, Architectures of split DdCBE_S, DdCBE, DdCBE-TOD6_S, and DdCBE-TOD6 at the C-terminal of N29 or N94 for E. coli-based editing assays. b, The editing efficiency of N29 and N94 splits in E. coli. Base editors were induced with 0.2 g/L arabinose for 1 h. c, The editing efficiency of N-half and C-half components of the N29 and N94 splits in E. coli. DdCBE and DdCBE-TOD6 share the same C-half split. Base editors were induced with 0.2 g/L arabinose for 1 h. d, Schematic representation of DdCBE_N29 and DdCBE-TOD6_N29 for mtDNA editing. e, f, The mtDNA editing efficiencies of DdCBE_N29 and DdCBE-TOD6_N29 at the MT-CYB (e) and MT-TS2 (f) site in HEK293T cells. The transfection duration was 3 days. g, h, The editing efficiency of DdCBE-TOD6_N67 split and its N-half and C-half components in E. coli. DdCBE-TOD6 variants were induced with 0.2 g/L arabinose for 1 h (g) or 3 h (h). Data are presented as mean ± SD from n = 3 independent experiments. Source data are provided as a Source Data file.
Extended Data Fig. 8 Editing activities of DdCBE-TOD6_N67-derived constructs for mtDNA.
a, Architectures of DdCBE and DdCBE-TOD6_N67-derived constructs. b–d, mtDNA editing efficiencies of DdCBE and DdCBE-TOD6_N67-derived constructs at the MT-CYB and MT-TS2 sites in HEK293T cells. Data are presented as mean ± SD from n = 3 independent experiments. Source data are provided as a Source Data file.
Extended Data Fig. 9 DdCBE-TOD6-derived constructs achieve single-nucleotide precision editing at non-CC context positions.
a–e, mtDNA editing efficiencies of DdCBE_N94 and DdCBE-TOD6-derived constructs at the MT-CO3 (a), MT-ND5 (b), MT-ND1.1 (c), MT-ND1.2 (d) and MT-ND1.3 (e) sites in HEK293T cells. Note that MT-ND5 site contains a CCCC sequence, and MT-ND1.3 site contains an ACC sequence. f–h, mtDNA editing efficiencies of DdCBE_N94 and DdCBE-TOD6-derived constructs at the mt-Nd1.1 (f), mt-Nd1.2 (g) and mt-Nd6 (h) sites in NIH/3T3 cells. Data are presented as mean ± SD from n = 3 independent experiments. The transfection duration was 3 days. i, Distribution of disease-associated mtDNA mutations across different sequence contexts. Source data are provided as a Source Data file.
Extended Data Fig. 10 DdCBE-TODs achieve high precision mtDNA editing in human primary skin fibroblast and MEF cells.
a, Average mtDNA genome-wide C•G to T•A conversion rate induced by DdCBE-TOD6_sCUTV and DdCBE-TOD6_dNU for MT-TK.2 sites in human primary skin fibroblasts. Naturally occurring single nucleotide variations (SNVs) with heteroplasmy fraction >10% were excluded from the analysis. Data represent the mean from n = 2 independent experiments. b, m.G8369 mutation load in untreated and DdCBE-TOD6_sCUT-treated MEF clones. Each dot represents the mutation load measured from a single-cell-derived clone. Successfully edited MEF clones by DdCBE-TOD6_sCUT exhibited mutation loads ranging from 42.01% to 89.89%, with a median mutation load of 70.05%. Median with interquartile range is shown, n = 4 for untreated, n = 53 for DdCBE-TOD6_sCUT. c, Heat map showing C•G-to-T•A conversion frequencies for 4 untreated clones and 4 clones with the highest mutation loads after DdCBE-TOD6_sCUT treatment, as depicted in panel (b). Shown are individual values. Source data are provided as a Source Data file.
Supplementary information
Supplementary Information
Supplementary Figs. 1–5, Sequences 1–3 and Note
Supplementary Table 1
MitoTALE binding sites.
Supplementary Table 2
Primers used in this paper.
Source data
Source Data Fig. 4
Statistical source data.
Source Data Fig. 5
Statistical source data.
Source Data Fig. 6
Statistical source data.
Source Data Extended Data Fig. 1
Statistical source data.
Source Data Extended Data Fig. 2
Statistical source data.
Source Data Extended Data Fig. 3
Unprocessed gels.
Source Data Extended Data Fig. 5
Statistical source data.
Source Data Extended Data Fig. 6
Statistical source data.
Source Data Extended Data Fig. 7
Statistical source data.
Source Data Extended Data Fig. 8
Statistical source data.
Source Data Extended Data Fig. 9
Statistical source data.
Source Data Extended Data Fig. 10
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mi, L., Li, YX., Lv, X. et al. Computational design of a high-precision mitochondrial DNA cytosine base editor. Nat Struct Mol Biol 32, 2575–2586 (2025). https://doi.org/10.1038/s41594-025-01714-2
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41594-025-01714-2
This article is cited by
-
Expansion of artificial intelligence for genome editing
Nature Structural & Molecular Biology (2025)