Abstract
Crohn’s disease pathology is modeled in TNFΔARE+/− mice that overproduce tumor necrosis factor (TNF) to drive disease through TNF receptors. An alternative ligand for TNF receptors, soluble LTα3, is produced by B cells, but has received scarce attention because LTα also partners with LTβ to generate membrane-tethered LTαβ2 that promotes tertiary lymphoid tissue—another feature of Crohn’s disease. We hypothesized that B cell-derived LTαβ2 would critically affect ileitis in TNFΔARE+/− mice. However, whereas deleting LTβ in B cells was essential for tertiary lymphoid tissue, disease pathology was minimally affected. By contrast, loss of B cell-derived LTα increased intestinal permeability, shrunk the pool of IgA+ ileal plasma cells, elevated cytokines and prompted weight loss, including loss of muscle mass—a systemic feature of Crohn’s disease. Neutralizing antibodies to LTα3 strongly augmented the cachexic-like effects of TNF. Thus, B cell-produced LTαβ2 and LTα3 have distinct roles in ileitis, with the role of LTα3 unexpectedly protective through counterbalancing TNF.
Similar content being viewed by others
Main
Transmural intestinal inflammation characterizes Crohn’s disease (CD)—a principal form of inflammatory bowel disease (IBD)1. Many patients with CD present with accompanying systemic manifestations, such as a low body mass index2. In the gastrointestinal tract, CD involves all layers of the intestine, from the mucosal surface to the underlying mesentery, and most frequently affects the ileum1. Early studies referred to the disease as regional ileitis that exhibited striking alterations in the lymphatic vasculature that were implicated in disease pathology3,4. Recently, we and others have identified that B cell-rich tertiary lymphoid structures (TLSs) arise adjacent to, or within, remodeled mesenteric lymphatic collecting vessels draining the ileum in patients with ileal CD and in the TNFΔARE mouse model of ileitis5,6,7,8. In TNFΔARE mice, deletion of the AU-rich response element (ARE) prolongs the half-life of TNF mRNA9. Increased tumor necrosis factor (TNF), whose expression is initiated by signals from the microbiome, promotes ileitis9,10,11.
Lymphotoxin (LT) is a TNF superfamily member required for lymph node (LN) and TLS generation and organization12,13 when it is expressed as a membrane-bound heterotrimeric (LTαβ2) complex that binds to LTβR on stromal cells and myeloid cells14. Often less considered is the soluble homotrimeric form LTα3. LTα3 binds to TNF receptors 1 (TNFR1) and TNFR2. Linked centrally to inflammation, LTα3 has been viewed as being the less potent ligand of TNFRs compared with TNF itself15,16,17,18,19,20. LTαβ2 forms readily to consume some of the available LTα when LTβ is coexpressed in activated B cells and T cells21.
Recent studies have implicated B cells in the pathogenesis of IBD, including CD22. B cells are especially enriched in TLSs of humans and mice5,7, leading us to consider that B cells may play a pivotal role in TLS development in ileitis. Furthermore, secretory immunoglobulin (sIg), synthesized by gut-resident plasma cells mainly as dimeric IgA, shifts towards IgG production in patients with CD, who often have an aberrant antimicrobial antibody responses that may contribute to the disease process23,24,25. Previous studies reported that B cell deletion in the TNFΔARE/+ mouse model did not prevent inflammation in the ileum of TNFΔARE/+ mice26. However, other features of the disease, such as TLS formation, lymph flow disturbances or weight loss were not reported. Here, we reexamined the role of B cells and LT production in ileitis.
Results
Absence of B cells moderately worsens local ileal disease
We first crossed TNFΔARE/+ mice to B cell-deficient μMT mice. These mice bred poorly, so we used a bone marrow (BM) chimeric approach wherein we crossed TNFΔARE/+ and μMT-TNFΔARE/+ mice to a CD45.1 congenic background and transplanted this marrow into CD45.2 congenic recipient mice (Fig. 1a). We quantified ileal inflammation in the recipients 16 weeks after BM transplant (BMT). Histologically, μMT-TNFΔARE/+ BM chimeras displayed a mildly increased disease score (Fig. 1b,c). Fecal lipocalin-2 (ref. 27) was similarly elevated in TNFΔARE/+ and μMT-TNFΔARE/+ BM chimeric mice (Fig. 1d). Using flow cytometry, we confirmed that B cells were absent in the blood (Extended Data Fig. 1a) and the ileum (Fig. 1e) in μMT-TNFΔARE/+ chimeras. Although IgA+ plasma cells were reduced in μMT-TNFΔARE/+ chimeras, they were not entirely absent (Fig. 1f), in part because some radioresistant plasma cells of recipient genotype were retained (Extended Data Fig. 1b)28. Flow cytometry also revealed that neutrophil (Fig. 1g) and T cell (Fig. 1h) numbers in the ileum were elevated in TNFΔARE/+ cohorts. For T cells, this elevation reached statistical significance only in μMT-TNFΔARE/+ chimeras (Fig. 1h). At least 70% of T cells in the ileum were CD4+ (Fig. 1i), and most of these were CD44hi CD62Llo effector/effector memory T cells (Fig. 1j). In TNFΔARE/+ BM chimeras, whether B cells were present or absent, a higher frequency of CD4+ T cells were FoxP3+ T regulatory cells (Fig. 1k) with similar proportions of FoxP3+RORγτ+ (Extended Data Fig. 1c). In the blood or proximal small intestine, neutrophils were not increased (Extended Data Fig. 1d,e), but monocytes were increased in TNFΔARE/+ or μMT-TNFΔARE/+ chimeras compared to wild type (WT) (Extended Data Fig. 1f), with a shift toward elevated Ly6C− nonclassical monocytes (Extended Data Fig. 1g). Taken together, ileal inflammation was moderately increased in the absence of B cells.
WT, TNFΔARE/+ and μMT-TNFΔARE/+ BM chimeras were analyzed 16–17 weeks post-BMT. a, Schema describing BM chimera groups. b, Representative hematoxylin and eosin staining of the distal ileum of mice given WT, TNFΔARE/+ and B cell-deficient μMT-TNFΔARE/+ BM. Scale bars: 150 μm. c, Semiquantitative histological scoring on the distal ileum of BM chimera recipients (three independent experiments (WT (n = 9), TNFΔARE/+ and μMT-TNFΔARE/+ (n = 14)). d, Fold change in the amount of lipocalin-2 in the stool of mice normalized to the average amount per WT group per experiment (three independent experiments, WT (n = 14), TNFΔARE/+ and μMT-TNFΔARE/+ (n = 13)). e–k, Flow cytometry on single-cell suspensions of ileum to analyze the numbers and proportions of various immune cells including numbers of CD19+ cells (e), IgA+ plasma cells (f), neutrophils (g) and T cells (h), or the proportion of total CD4+ T cells (i), effector memory CD4+ T cells (j) or regulatory CD4+ T cells (k) (three independent experiments, WT (n = 15), TNFΔARE/+ and μMT-TNFΔARE/+ (n = 14)). Gating strategies for flow cytometry shown in Supplementary Figs. 1 and 3. All dataplots show mean ± s.e.m. Each symbol represents one mouse. One-way ANOVA with post hoc Tukey test (c), Kruskal–Wallis test with Dunn’s correction (d–k).
B cell deficiency restores mesenteric lymph flow
The ileum-draining mesenteries of TNFΔARE/+ BM chimeras but not WT BM chimeras (Fig. 2a) exhibited B cell-rich TLSs (Fig. 2b, arrows show B cell-rich lymphoid tissue). As expected, B cell-rich follicular structures were not present in μMT-TNFΔARE/+ BM recipients (Fig. 2c). With or without B cells, however, intense CD115+ myeloid cell-rich mesenteric inflammation emerged (Fig. 2b,c; green staining) marked by accumulation of neutrophils (Fig. 2d) and MHC II+CD64+ macrophages (Fig. 2e), with macrophage numbers statistically significantly higher in μMT-TNFΔARE/+ chimeras (Fig. 2e).
WT, TNFΔARE/+ and μMT-TNFΔARE/+ BM chimeras 16–17 weeks post-BMT were studied. a–c, Representative whole-mount images (LYVE-1 red, CSF1R green, B220 blue) staining mice that received WT (a), TNFΔARE/+ (b) or μMT-TNFΔARE/+ (c) BM. Scale bars: 500 μm. d,e, The number of Ly6G+ neutrophils (d) and CD64+ MHCII+ macrophages (e) in the mesentery of TNFΔARE/+ and μMT-TNFΔARE/+ BM chimeric mice (three independent experiments, WT and TNFΔARE/+ (n = 14) and μMT-TNFΔARE/+ (n = 13)). f–h, Representative stereoscope images (left, brightfield and FITC channels; right, FITC channel alone) of the mesentery of anesthetized mice after FITC–dextran injection of 1–1.5 μl into the most distal Peyer’s patch. Mice given WT (f), TNFΔARE/+ (g) and μMT-TNFΔARE/+ (h) BM. Scale bars: 1.5 mm. i, Quantification of time to MLN in tracer experiments (three independent experiments, WT (n = 4), TNFΔARE/+ (n = 6) and μMT-TNFΔARE/+ (n = 4)). j–l, Number of TCRβ+ T cells (j), CD19+ B cells (k) and IgA+ plasma cells (l) in the mesentery of TNFΔARE/+ and μMT-TNFΔARE/+ BM chimeric mice (three independent experiments, WT and TNFΔARE/+ (n = 14) and μMT-TNFΔARE/+ (n = 13)). Gating strategy for flow cytometry shown in Supplementary Fig. 1. All dataplots show mean ± s.e.m. Each symbol represents one mouse; Kruskal–Wallis test with Dunn’s correction for d, e and i–l.
To evaluate lymphatic outflow through the ileum-draining mesentery, we injected ~1.5 μl of 2,000 kDa fluorescent dextran into the most distal Peyer’s patch7. In WT chimeric mice, the tracer rapidly entered mesenteric collecting lymphatic vessels, reaching the mesenteric LN (MLN) within 5 min (Fig. 2f–i and Supplementary Video 1). However, in five of six TNFΔARE/+ chimeras, lymphatic flow to the MLN was blocked (Fig. 2g,i and Supplementary Video 2) with lymphatic leak observed in the one mouse that retained flow (Supplementary Video 3). By contrast, lymphatic flow to the MLN was intact in all μMT-TNFΔARE/+ chimeric mice (Fig. 2h,i and Supplementary Video 4), indicating that B cells, although not required for mesenteric inflammation, mediated obstruction of lymph outflow from the ileum. T cell numbers increase in the mesentery of TNFΔARE/+ mice7. However, here, although they were elevated in TNFΔARE/+ BM chimeras with or without B cells, the differences did not reach statistical significance (Fig. 2j). This may be due to several factors, including a technical issue that, for flow cytometry, we processed the entire mesentery—including regions draining the relatively uninflamed proximal small intestine—diluting out T cells in the ileum. CD19+ B cells were, as expected, absent in the mesentery of WT BM chimeras (Fig. 2k), with a correspondingly strong reduction in IgA+ plasma cells (Fig. 2l).
Collectively, these findings indicate that B cells orchestrate blocked outflow of ileal lymph in TNFΔARE/+ mice, possibly by promoting B cell-rich TLS aggregates. However, neither the absence of B cells nor the associated restoration of lymph flow protected the mesentery from a myeloid-rich inflammatory response. Mesenteric inflammation was instead elevated.
Absence of B cells augments ileitis-associated weight loss
Many patients with CD are underweight or have reduced muscle mass2. TNFΔARE/+ mice were often growth stunted. Our use of BM chimeras, in which all recipients were WT mice that developed normally to adulthood before BMT, allowed for a rigorous approach to study any alterations in body composition in response to ileitis disconnected from developmental problems. WT BM chimeras increased body weight by 17% during the 2–16 weeks after BMT (Fig. 3a) In contrast, TNFΔARE/+ BM chimeras initially lost weight before experiencing a moderate increase that plateaued 7 weeks later, such that these mice had only a mean 6% increase from their initial weight (Fig. 3a). By comparison, μMT-TNFΔARE/+ BM chimeras lost 2% body weight over the 16-week period (Fig. 3a and Extended Data Fig. 2a). This weight loss required the inflammatory context provided by the TNFΔARE/+ genotype, as μMT BM donors on a WT background increased their weight normally (Fig. 3a). Magnetic resonance imaging (MRI)-based body composition analysis revealed that, with low fat mass in all TNFΔARE/+ mice, lean mass especially declined in μMT-TNFΔARE/+ BM chimeras (Fig. 3b).
a, Weekly percent weight change in mice given WT, μMT, TNFΔARE/+ or μMT-TNFΔARE/+ BM normalized to weight 2 weeks post-BMT (six independent experiments, WT (n = 24), μMT (n = 9), TNFΔARE/+ (n = 22), μMT-TNFΔARE/+ (n = 23)). b–f, Mice given WT, TNFΔARE/+ and μMT-TNFΔARE/+ BMT after 14 weeks (three independent experiments, WT and TNFΔARE/+ (n = 14) and μMT-TNFΔARE/+ (n = 13)). b, EchoMRI evaluation of the body composition of mice given WT, TNFΔARE/+ or μMT-TNFΔARE/+ BM. c, Core body temperature of mice given WT, TNFΔARE/+ or μMT-TNFΔARE/+ BM. d–f, Mice were housed in metabolic cages for 24 h, and activity (d), heat (e) and food intake (f) were measured. g, Weight of the gastrocnemius muscle in BM recipients (three independent experiments, WT (n = 13), TNFΔARE/+ (n = 14) and μMT-TNFΔARE/+ (n = 12)). h, Micro-CT analysis of paws of the bone mineral density (three independent experiments WT and μMT-TNFΔARE/+ (n = 14), TNFΔARE/+ (n = 13)). All data represent mean ± s.e.m. One-way ANOVA with post hoc Tukey test in c, g and h; two-way ANOVA (to test whether light versus dark is different in addition to whether there are group differences) in d–f.
Data from metabolic cages revealed that μMT-TNFΔARE/+ BM chimeras had lower core body temperature compared to controls (Fig. 3c), making it unlikely that the observed weight difference in μMT-TNFΔARE/+ BM chimeras was driven by brown adipocyte thermogenesis, as the core temperature of mice undergoing thermogenesis would not decrease. Mice are nocturnal and most active during the dark phase; however, TNFΔARE/+ BM chimeric mice were significantly less active in the dark phase than controls (Fig. 3d). Energy expenditure (heat) decreased in both TNFΔARE/+ and μMT-TNFΔARE/+ chimeric mice in the light and dark phases compared to WT controls (Fig. 3e and Extended Data Fig. 2b), with the effect more pronounced in μMT-TNFΔARE/+ chimeras. Contrary to our expectations, food intake in the dark phase was increased in μMT-TNFΔARE/+ chimeric mice, indicating that food intake could not explain the weight deficit (Fig. 3f and Extended Data Fig. 2c). There were no differences in water intake (Extended Data Fig. 2d) or whole-body hydration (Extended Data Fig. 2e). Upon tissue harvest, the gastrocnemius muscle weighed less in μMT-TNFΔARE/+ chimeras compared to the other groups (Fig. 3g). Bone mineral density in the paws of the mice was similar between groups (Fig. 3h). TNFΔARE/+ mice, in addition to ileitis, also develop rheumatoid arthritis-like disease in their joints9. Without B cells, bone porosity decreased (Extended Data Fig. 2f), and bone surface to bone volume, indicative of bone pitting, increased (Extended Data Fig. 2g), indicative of worsened arthritis. These data collectively indicate that systemic features of ileitis, including weight loss and reduced muscle mass as well as arthritis, were exacerbated without B cells.
Luminal IgA reduces ileitis without impacting body weight
To delineate the role of B cells in ileitis, we sought to understand the role of sIg, as B cell deficiency would eliminate IgA transported to the intestinal lumen in TNFΔARE-driven ileitis. Polymeric immunoglobulin receptor (pIgR) expression on intestinal epithelial cells translocates IgA into the lumen, where secretory IgA binds and neutralizes some microbes29. We thus lethally irradiated Pigr−/− mice or Pigr+/+ littermates, and reconstituted both genotypes with TNFΔARE/+ BM, separately housing the groups afterwards. We confirmed immunostaining for IgA was high on the mucosal surface of WT ileum (Fig. 4a) but trapped at the abluminal border in Pigr−/− littermates (Fig. 4b; yellow arrowhead). The latter had low coverage of IgA on the ileal surface compared with WT (Fig. 4a,b), but IgA was detected in a spike-like fashion in the ileal lumen of some Pigr−/− mice (Fig. 4b), reminiscent of the morphology of segmented filamentous bacteria, suggesting that segmented filamentous bacteria is partly regulated by pIgR-mediated transcytosis of IgA30.
All panels show lethally irradiated Pigr−/− mice or Pigr+/+ mice given TNFΔARE/+ BMT for 16 weeks. a,b, Visualization of two projections (left, en face; right, cross-sectional) of a 3D rendering of ileum after whole-mount staining for IgA (red), smooth-muscle actin (white) and DAPI to identify nuclei (blue). a, Representative Pigr+/+ recipient mouse given TNFΔARE/+ BM. b, Representative Pigr−/− recipient mouse given TNFΔARE/+ BM; yellow arrow, abluminal accumulation of IgA in the Pigr−/− recipient. Scale bars: 100 μm. c, Micrograms of IgA per gram of stool in Pigr−/− versus Pigr+/+ recipients given TNFΔARE/+ BM. d, Fecal lipocalin-2 levels normalized to the average fecal lipocalin-2 amount in the Pigr+/+ TNFΔARE/+ BM controls per experiment. (two independent experiments, Pigr+/+ (n = 7), Pigr+/+ (n = 9). e,f, Flow cytometry on the ileum shows numbers of neutrophils (e) and T cells (f) (three independent experiments, Pigr+/+ (n = 8), Pigr−/− (n = 12)). g, Total body weight (four independent experiments, Pigr+/+ (n = 12), Pigr−/− (n = 15)). h, Number of monocytes in the blood (three independent experiments, Pigr+/+ (n = 8), Pigr−/− (n = 12)). i, Number of TLSs along the most distal branch of the mesentery (one experiment, Pigr+/+ (n = 3), Pigr−/− (n = 6)). Gating strategy for flow cytometry in Supplementary Figs. 1 and 2. All data in c–i show one mouse per symbol and depict mean ± s.e.m. Two-tailed Mann–Whitney test for all panels.
Stool IgA was greatly reduced in Pigr−/− versus Pigr+/+ hosts (Fig. 4c). No difference in fecal lipocalin-2 existed between the groups (Fig. 4d). However, in the ileum lamina propria, loss of host-expressed Pigr−/− increased infiltrating neutrophils (Fig. 4e) and T cells (Fig. 4f) in TNFΔARE/+ chimeric mice (Fig. 4f), indicating that sIg protected against ileal inflammation. This increase in intestinal inflammation was not accompanied by a difference in body weight (Fig. 4g), contrasting with the impact of overall B cell deficiency (Fig. 3). The number of monocytes in the blood was similar between groups (Fig. 4h), and the number of TLSs in the ileal-draining mesentery was also similar in the two genotypes (Fig. 4i). Thus, B cells protect against ileitis through luminal secretion of sIg such as IgA. However, although this mechanism protected locally against inflammation, B cell-dependent roles in body weight and mesenteric TLS formation were independent of sIg.
Distinct phenotypes arise in Lta-deficient versus Ltb-deficient B cells
We next tested whether B cell synthesis of LTs in the TNFΔARE/+ model governed TLS formation and whether, in turn, loss of TLSs explained the role of B cells in the failure to maintain body mass. B cell-selective loss of LTα would eliminate expression of both LTα1β2 that binds LTβR and LTα3 that binds TNFR1 and TNFR2 with high affinity and HVEM with lower affinity31. Since the genes for TNF, LTα and LTβ are closely clustered32, crossing LTα knockout (KO) or LTβ KO mice to TNFΔARE mice was not tractable. Instead, mixed BM chimeras where 90% of the donor BM was provided by μMT-TNFΔARE/+ donor marrow and 10% of the BM was supplied by either WT, Lta−/− or Ltb−/− donors were established. B cells could only be supplied by BM that is not from the μMT-TNFΔARE/+ source and thus would yield WT, Lta−/− or Ltb−/− B cells in the different cohorts. By contrast, all hematopoietic cells except B cells would arise primarily (~90%) from the μMT-TNFΔARE/+ BM. The use of BM mixtures rather than 100% BM of a TNFΔARE/+ genotype decreased the pace of disease development, so that TLSs required >25 weeks to arise reliably. The duration of these experiments was extended accordingly.
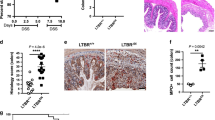
At 30–32 weeks post-BMT, approximately 20 TLSs arose in the most distal ileal-draining mesentery of WT/μMT-TNFΔARE/+ chimeras (Fig. 5a). These TLSs were enwrapped by lymphatics7 (Fig. 5b), and their en face area averaged 4.2 × 104 μm2 (Fig. 5c). However, Lta−/−/μMT-TNFΔARE/+ or Ltb−/−/μMT-TNFΔARE/+ chimeric mice, bearing B cell-selective loss of Lta or Ltb−/− respectively, had few ileal mesenteric TLSs (<2) (Fig. 5a), and these were morphologically undeveloped with minimal lymphatics (Fig. 5b) and about half the surface area (average of 2.5 × 104 μm2) of controls (Fig. 5c).
Chimeras were studied at 30–32 weeks after BMT, wherein WT recipient mice received μMT-TNFΔARE/+ BM at 90% and 10% marrow from WT, Lta−/− or Ltb−/− mice to resupply the B cell compartment with these respective genotypes. a, Quantification of the number of TLSs in the branch of the mesentery that drains the distal ileum in these chimeras where B cells were WT, Lta−/− or Ltb−/−. Three independent experiments, WT B cells (n = 13), Lta−/− B cells (n = 8) and Ltb−/− B cells (n = 5). Statistical significance was evaluated using Kruskal–Wallis test. b, Representative whole-mount images (LYVE-1 red, CSFR1 green, B220 blue) of lymphoid aggregates in the ileal associated mesentery of WT/μMT-TNFΔARE/+ or Ltb−/−/μMT-TNFΔARE/+ chimeric mice. Scale bar: 500 μm. c, TLS area was assessed in whole-mount images and the area of each TLS quantified depicted in the graph, with each symbol representing one TLS from WT/μMT-TNFΔARE/+ (n = 4 mice evaluated) or Ltb−/−/μMT-TNFΔARE/+ (n = 5 mice evaluated) chimeric mice. Statistical significance used unpaired t test with Welch’s correction for nonparametric distribution. d, Flow cytometry on single-cell suspensions of the distal ileum of mice that received WT/μMT-TNFΔARE/+, Lta−/−/μMT-TNFΔARE/+ or Ltb−/−/μMT-TNFΔARE/+ BM to quantify neutrophils and T cells. Four independent experiments were done, WT/μMT-TNFΔARE/+ (n = 19), Lta−/−/μMT-TNFΔARE/+ (n = 9), Ltb−/−/μMT-TNFΔARE/+ (n = 10). e, Eight weeks after TNFΔARE/+ BMT, mice were dosed weekly with isotype control or LTβR-Fc for an additional 8 weeks in one experiment, isotype and LTβR-Fc (n = 3 mice in each cohort). TLSs were quantified in TNFΔARE/+ BM recipients given LTβR-Fc in the distal mesentery or isotype control. Flow cytometry on the ileum of mice given TNFΔARE/+ BM and treated with LTβR-Fc or isotype control to quantify neutrophils. f, Weekly percent weight change in μMT-TNFΔARE/+ BM chimeras that had WT (n = 13), LTα−/− (n = 12) or LTβ−/− (n = 10) B cells. Data are normalized to mouse weight 2 weeks post-BMT. Statistical analysis was carried out using two-way ANOVA with Tukey’s multiple comparisons post hoc test. Actual body weights at the endpoint are graphed separately on the right, with statistical analysis carried out using one-way ANOVA, with Tukey’s test for multiple comparisons. g, Relative intestinal permeability to FITC–dextran administered by gavage and assessed 2 h later (two independent experiments, WT B cells (n = 5), Lta−/− B cells and Ltb−/− B cells (n = 5)). h,i, Plasma (h) and fecal (i) IgA levels measured on mice given WT BM or mice lacking Lta−/− or Ltb−/− (two independent experiments, WT B cells (n = 8), Lta−/− B cells (n = 4), Ltb−/− B cells (n = 5)). j, Flow cytometry on single cell-digests of the ileum to quantify IgA+ plasma cells from the same mice used to generate data in d. k,l, Representative images of the mid-villus region in the ileum viewed in whole-mount specimens from en face after staining for IgA (red), smooth-muscle actin (white) and DAPI to identify nuclei (blue) (k) and quantification of their frequency (l). Two independent experiments, WT B cells (n = 5), Lta−/− B cells and Ltb−/− B cells (n = 5). Statistical comparisons used one-way ANOVA with Tukeyʼs test for multiple comparisons. Gating strategy for flow cytometry shown in Supplementary Fig. 1. Graphic plots show one mouse per symbol and depict mean ± s.e.m.
Between cohorts, neutrophil and T cell infiltration into the ileum was similar (Fig. 5d). Like the genetic manipulations, treatment of TNFΔARE/+ BM chimeric mice with LTβR-Ig for the last 8 weeks of a 16-week period to block LTβR signaling inhibited TLS but not neutrophil infiltration (Fig. 5e). The ability to gain weight in a sustained manner after BMT was impaired in mixed TNFΔARE/+ BM chimeric mice wherein B cells lacked Lta, whereas loss of Ltb was unimpactful (Fig. 5f), as was LTβR-Ig treatment (Fig. 5e). Furthermore, intestinal permeability increased in mice bearing Lta−/− but not Ltb−/− B cells (Fig. 5g). Total plasma IgA trended toward being elevated when B cells lacked Lta (Fig. 5h) whereas fecal IgA was similar (Fig. 5i). However, there were significantly fewer IgA+ plasma cells within the ileum when quantified by flow cytometry (Fig. 5j) or by three-dimensional (3D) imaging of villi in the ileum (Fig. 5k,l). Thus, systemic sources of IgA persisted, and these could access the gut lumen, but local IgA+ cells were lost in chimeras carrying Lta−/− B cells, distinguishing this pattern from that in Pigr−/− hosts.
Neutralizing LTa3 promotes TNF-driven weight loss
To further evaluate a possibly distinct role for B cell-expressed LTα compared with LTβ, as the data so far suggested, we turned to Fc-mutant anti-LTα3 mAb (S5H3; LT-α.Fc-MT) that cannot engage FcRs but that binds and neutralizes LTα3 (ref. 33). In contrast to the LTβR-Ig fusion protein that inhibits LTαβ2–LTβR binding and prompts disorganization of lymphoid tissue, anti-LTα3 mAb efficiently blocks LTα3-TNFR2 interactions but poorly affects LTαβ2-LTβR interactions and does not induce lymphoid tissue disorganization33. In our studies, TNFΔARE/+ BM chimeras given this anti-LTα3 mAb lost over 5% of their body weight in 2 days, whereas the weight of WT mice receiving anti-LTα3 mAb remained steady (Fig. 6a). In the short time frame of a 2-day treatment period, and resembling results from BM chimeric mice wherein Lta or Ltb was genetically deleted in B cells, LTβR-Ig treatment for 2 days disrupted germinal center CD21/CD35 staining in the splenic white pulp (Extended Data Fig. 3a,b), but such staining remained intact in mice treated with anti-LTα3 mAb (Extended Data Fig. 3b), confirming that the anti-LTα3 mAb did not block LTβR signaling in our hands.
a–e, Percent weight change (a) or quantification of cytokines (b–e) including TNF (b), LTα (c), IL-33 (d) and IL-1β (e) of mice comparing day 0 to day 2 in mice transplanted with WT or TNFΔARE/+ BM 9 weeks post-BMT, then dosed with anti-LTα3 antibody or only isotype. f, Percent weight change, comparing day 0 to day 2 in mice transplanted with WT or TNFΔARE/+ BM 9 weeks post-BMT, then dosed with anti-LTα3 antibody, anti-TNF antibody, both or isotype only. g–i, Hydration analysis (g), activity (h) and food intake in the same mice on day 3 (i) dosed again on day 2 after weight assessment were made. j, MRI measurements of body fat composition were also made on these mice on day 3. k, Weights of the gastrocnemius muscle on day 5—the experimental endpoint. g–i, All plots depict mean ± s.e.m. One symbol represents data from one individual mouse. Three independent experiments for a, n = 10 for WT mice and n = 13 for TNFΔARE/+ BM recipients. Two independent experiments for b–k, n = 8 per group. Two-way ANOVA with post hoc Tukey test was used for a–e, h and i to determine whether there was a difference between WT and TNFΔARE/+ mice as well as between isotype and anti-LTα3 treated mice. A one-way ANOVA with post hoc Tukey test was used for f, g, j and k. In all graphs except for c, the P value depicts the results of the post hoc test; in c, the P value shown is the difference between all WT mice and all TNFΔARE/+ BM recipients.
To better understand how neutralizing LTα3 affected weight loss only when TNF was present, we quantified ileal cytokines. Anti-LTα3 treatment augmented TNF levels in the ileum in TNFΔARE BM chimeric mice but not in WT counterparts (Fig. 6b). The concentration of LTα (LTα3 and LTαβ2 combined) was higher than that of TNF in the ileum (Fig. 6c) and unaffected by treatment with anti-LTα3 mAb (Fig. 6c). Other cytokines elevated in the ileum of TNFΔARE chimeras were IL-33 (Fig. 6d) and IL-1β (Fig. 6e). These were unaffected by anti-LTα3 mAb treatment (Fig. 6d,e).To further probe whether the impact of the anti-LTα3 mAb on weight loss relied on TNF itself, we studied TNFΔARE/+ BMT mice treated with either (1) isotype mAb only, (2) anti-TNF mAb, (3) anti-LTα3 mAb or (4) combined anti-TNF and anti-LTα3 mAbs. TNFΔARE/+ BM recipients given anti-LTα3 again rapidly lost more than 5% of their initial body weight within 2 days, whereas isotype-treated and anti-TNF treated TNFΔARE/+ BM recipients maintained their weight. There was also a partial protection against weight loss in mice given both anti-TNF and anti-LTα3 (Fig. 6f), suggesting the biological impact of anti-LTα3 was connected to the presence of TNF. During the treatment regimen, which continued through day 5, we transitioned these four groups of mice to metabolic cages between days 3 and 5. No difference was observed in hydration ratio (Fig. 6g). Changes in activity (Fig. 6h) or food intake (Fig. 6i) also did not explain the weight loss. Just before transitioning to the metabolic cages, the four groups of mice were analyzed by EchoMRI. Anti-LTα3 treated mice had a significant reduction in fat mass (Fig. 6j). Upon euthanasia at day 5, muscles were collected, and the mass of the gastrocnemius muscle was found to be also reduced in TNFΔARE/+ BM-bearing mice treated with anti-LTα3 (Fig. 6k). In separate experiments, nutrient absorption assessed by fecal bomb calorimetry was unaffected by loss of B cell Lta or neutralization with anti- LTα3 (Extended Data Fig. 4a,b). Similarly, challenge with an oral bolus of triglyceride uncovered no alterations in fat absorption in steady state (Extended Data Fig. 4c) or in ileitis (Extended Data Fig. 4d). These data suggest that LTα3 protects against inflammation-mediated weight loss in a TNF-driven model of ileitis and that LTα3 holds back production of TNF in an environment already poised for producing TNF—a cytokine that has been associated historically with a cachexic response that includes heightened weight loss.
Contrasting roles of LTα3 and TNF in sustaining B cells in ileitis
In contemplating how LTα3 and TNF might be interlinked in ileitis and related to B cell biology, as the data above suggested, we considered that B cells induce these two cytokines during immunological activation34,35,36. Indeed, these cytokines act partly autologously during B cell activation, proliferation and antibody production35,37,38,39. In the mesentery of patients with ileal CD, the number of B cells in regions of the mesentery that drained the actively inflamed ileum was increased compared to control samples5 (Extended Data Fig. 5a), and scRNA sequencing analysis of CD45+ cells isolated from the mesentery enriched for B cells, especially from disease-active regions of patients with CD (Extended Data Fig. 5b). These cells expressed LTA and TNF transcripts (Fig. 7a). Nineteen clusters were separable on a uniform manifold approximation and projection (UMAP) plot (Extended Data Fig. 5c) and ascribed cell identity based on expression features (Extended Data Fig. 5d). Cells expressing LTA included naive B cells, memory B cells, plasma cells, several subpopulations of memory T cells and some plasmacytoid dendritic cells (DCs). All, except for plasmacytoid DCs, were also positive for TNF, while macrophages and other DCs (cDC1 and cDC2) expressed TNF but minimal LTA (Fig. 7a). Cell types expressing LTA also expressed LTB at equal or higher levels and proportions. LTA+ B cells expressed genes encoding TNFR2, but not LTβR (Fig. 7a). These data confirm that, although macrophages most robustly express TNF among hematopoietic cells, B and T cells in CD are key coexpressers of LTA, LTB and TNF.
a, scRNA-seq of sorted live, CD45+ mesenteric cells from three patients with CD with matched noninflamed and inflamed draining mesenteric samples. The dotplot depicts average expression of genes in the TNF/LTα/LTβ family along with their receptors. A UMAP plot of mesenteric immune cells is depicted in Extended Data Fig. 5b,c. b, Distribution of TNF+CD45+ cells analyzed with no ex vivo stimulation after staining permeabilized cells in cell suspensions of the ileum, mesentery or draining mLN of BM chimeric mice for TNF. TNF+ cells were gated and then divided into cell types that were TNF+ to assess proportional contribution of each population to the fraction of TNF+ cells in the tissue. This does not account for amount of TNF produced per cell or the possibility that some TNF is secreted and some is membrane-anchored. The same three mice were studied across the different tissue sites. c,d, UMAP projections of scRNA-seq on mLN cells that had migrated from the ileum in the previous 20 h. Feature plots (c) depict key genes identifying T cells (Cd3e), B cells (Ighd), DCs (Fscn1) and expression patterns of Tnf, Lta and Ltb. Cluster names assigned on the basis of marker genes are labeled in d, with distinct UMAP projections depicting profiles obtained in WT or TNFΔARE/+ chimeras treated with WT or anti-LTα3 neutralizing mAb. e, Dotplot depicting average expression of genes in the TNF/LTα/LTβ family along with their receptors and other genes that were impacted by anti-LTα3 treatment in either of the two B cell clusters or in CD4+ T cells. f–i, Mixed BM chimeras wherein WT recipient mice received μMT-TNFΔARE/+ BM at 90% and 10% marrow from CD19Cre/+ × Tdtomatofl/fl mice or CD19Cre/+ × TNFfl/fl × Tdtomatofl/fl mice to resupply the B cell compartment with these respective genotypes and to mark CD19Cre active cells with TdTomato expression. f, Percentage of CD19+ B cells in the blood 10 weeks after BMT to assess CD45.2+ donor engraftment. g, Weekly percent weight change in chimeras that had received μMT-TNFΔARE/+/CD19cre/+Tdtomato/+ mixed BM (n = 5) or μMT-TNFΔARE/+/CD19cre/+Tdtomato/+TNFfl/fl (n = 5) B cells. Data were normalized to mouse weight 2 weeks post-BMT. Statistical analysis was carried out using two-way ANOVA with Tukey’s multiple comparisons post hoc test, showing that the curves were not statistically distinct. h,i, Percentage of CD19+ in the blood (h) or ileum (i) that were Tdtomato+ 32 weeks after BMT (one experiment μMT-TNFΔARE/+/CD19cre/+Tdtomato/+ (n = 5 at 10 weeks, n = 3 at 32 weeks), μMT-TNFΔARE/+/CD19cre/+Tdtomato/+TNFfl/fl (n = 5)). Flow cytometry gating strategies in Supplementary Figs. 1, 2 and 5. Graphs depict mean ± s.e.m. Two-tailed Mann–Whitney test was applied for statistical analyses in f, h and i.
To move from human to mice in considering the expression of these cytokines during ileitis, we stained permeabilized CD45+ cells for TNF in the ileum, underlying mesentery or draining mLN of TNFΔARE/+ mice. TNF+ B cells, especially CD19+IgD− antigen-experienced B cells, were detected at all sites, especially in the mLN and mesentery, with TNF production from both IgD− and IgD+ B cells (Fig. 7b). The principal immune cells producing TNF at the ileum were neutrophils and macrophages, with TCRβ−CD19− lymphocytes (possibly ILCs) also among the largest producers (Fig. 7b). As we were interested in characterizing B cells from both WT and TNFΔARE/+ mice and there were not as many B cells in the mesentery of WT mice (Fig. 2k), we selected the mLN as the tissue site where we might best characterize B cells expressing Lta, Ltb or Tnf. We profiled these cells in mLN from WT or TNFΔARE/+ BM chimeric mice that had received short-term treatment with anti-LTα3 neutralizing or isotype-matched mAb.
To optimize our evaluation of whether anti-LTα3 mAb affected B cells or other immune cells in the mLNs, we focused on immune cells that had mobilized from the intestine to mLN over the past day, allowing us to avoid gathering data on the many naive lymphocytes that entered the LN directly without having passed through the intestine. To do so, our evaluation used WT or TNFΔARE/+ BM chimeric mice that expressed KikGR to allow for phototracking40. A day before photoconversion of the ileum7, we administered anti-LTα3 or isotype control mAbs. We gave another dose upon surgical closure following photoconversion, harvested the mLNs 20 h later, sorted the converted KikRED cells from the mLN and subjected them to sc-RNAseq, pooling three mice per experimental cohort. The anti-LTα3 mAb did not affect total cell recovery, which could be separated into eight UMAP clusters (Extended Data Fig. 5e). Most migrating cells arriving in the mLN were IgD+ B cells that separated into two clusters (Fig. 7c), along with T cells (Cd3e+) and DCs (Fscn+). Ltb mRNA was broadly expressed among CD4+ T cells but was most enriched in B cell cluster 1. A subpopulation of both B and T cells expressed Lta mRNA (Fig. 7c). Lta and Tnf were coexpressed by many B cells, confirmed in scatter plot analysis (Extended Data Fig. 5f).
In vivo neutralization of LTα3 led to notable loss of Lta+ B cells and a corresponding transcriptional shift with many fewer cells observed in the UMAP space where Lta was most prominent (Fig. 7d). However, T cells, including Lta+ T cells, seemed unaffected (Fig. 7d,e). WT and TNFΔARE/+ chimeric mice showed reductions in Lta+ B cells upon treatment with anti-LTα3 mAb, but this change was most striking in TNFΔARE/+ chimeric mice (Fig. 7d,e). T cell expression of Lta was not reduced by anti-LTα3 (Fig. 7d,e), indicating that B cells were preferentially reduced. However, Tnf was expressed more highly in CD4+ T cells than in the B cells and, here, anti-LTα3 decreased Tnf expressed in both T and B cells (Fig. 7e). Tnfrsf1b2 (encoding TNFR2) and low levels of Tnfrsf14 (encoding HVEM) were expressed by B cells. T cells expressed these receptors and also expressed Tnfrsf1a (encoding TNFR1) (Fig. 7e). Neither B nor T cells expressed LTβR. Several mRNAs not expressed in T cells but present in B cells were downregulated by anti-LTα3 in WT and TNFΔARE/+ chimeras, including the IgE receptor Fcr2a (encoding CD23a), which marks follicular B cells, Cd83 (encoding CD83) and IL4i1 (Fig. 7e), which encodes an enzyme regulating tryptophan metabolites and thereby suppresses immune activation41. Bhlhe40 expressed by B cells was reduced in response to anti-LTα3 in WT and TNFΔARE/+ chimeras but in CD4+ T cells its expression was observed mainly in TNFΔARE/+ chimeras and was not altered by anti-LTα3 (Fig. 7e). C1qbp and Nr4a1 were expressed more highly in CD4+ T cells but also present in B cells, with reduced expression of both in response to anti-LTα3 (Fig. 7e). Reduced expression of Nr4a1 in CD4+ T cells, indicative of antigen activation status42, was especially marked (Fig. 7e). Igha encoding IgA was high in a fraction of the B cells in TNFΔARE/+ chimeric mice, and these were largely lost in response to LTα3 neutralization (Fig. 7e), reminiscent of the loss in IgA+ plasma cells in the ileum of mice wherein B cells lacked expression of the Lta gene (Fig. 5l).
The loss of IgA+ cells in TNFΔARE/+ chimeric mice with Lta−/− B cells at the ileum in earlier experiments and their loss herein after treatment with anti-LTα3 mAb (Fig. 7d) suggested that autologous LTα3 may support a subset of activated B cells that normally induce LTα3 and TNF during activation. We thus wondered whether the loss of Tnf expression in B cells in TNFΔARE/+ chimeras might prompt a comparable outcome as B cell autonomous loss of Lta, considering the concept that each can act in a cell autonomous manner35,38. We compared two groups of mixed BM chimeric mice wherein CD45.1 WT recipient mice were reconstituted with μMT-TNFΔARE/+ BM at 90% but differed by receiving either (1) 10% BM from CD19Cre × Tnffl/fl × TdTomatofl/fl that would mark Cre-activated deletion with Tomato expression and also delete Tnf or (2) 10% BM CD19Cre × TdTomatofl/fl that would mark Cre-activated deletion but B cells would still be able to express Tnf. Reconstitution of CD45.1 recipient mice with CD45.2+ donor BM was greater than 99%, as assessed in blood CD19+ B cells (Fig. 7f and Extended Data Fig. 6a). Differences in infiltrates of neutrophils and lymphocytes were not observed in the ileum (Extended Data Fig. 6b), resembling the outcome following B cell loss of Lta or Ltb. However, in contrast to B cell deletion of Lta or Ltb, loss of B cell Tnf allowed for TLSs of similar size and number to emerge (Extended Data Fig. 6c,d). Like Ltb deficiency in B cells, and in contrast to Lta deficiency in B cells, body weight was not affected by loss of B cell Tnf (Fig. 7g). The fraction of TdTomato+ cells was enhanced significantly in blood and ileal CD19+ B cells when Tnf was deleted (Fig. 7h,i), suggesting that cell autonomous expression of Tnf leads to loss of B cells. When these findings are considered alongside results observed upon loss of B cell Lta or following LTα3 neutralization, we conclude that expression of LTα3 versus TNF in B cells oppositely impacts B cell fate during ileitis. Extended Data Fig. 7 schematizes the collective findings we uncovered, highlighting the multifactorial role of B cells in ileitis.
Discussion
We examined the role of B cells in Crohn’s-like ileitis using the TNFΔARE/+ mouse model, wherein disease can be transferred through BMT. We were unexpectedly struck that the loss of B cells was associated with an inability to maintain body weight as disease progressed—a feature reflected in many humans with CD2,43. Furthermore, deletion of LTβ versus LTα in B cells gave rise to divergent results. The latter, but not the former, impaired weight gain during ileitis, suggesting that LTα3 but not LTαβ2 was active in governing body weight during ileitis. Ultimately, both genetic approaches and use of a neutralizing anti-LTα3 mAb pointed toward an apparent role for LTα3 in counterbalancing the action of TNF. LTα3 was, early on, viewed as a partial agonist of TNFRs15 and consistently showed more attenuated inflammatory effects in vitro than TNF on the same target cells16,17,18,19,20,44. Thus, a possibility exists that when the two cytokines are found in the same setting, LTα3 offsets some of the action of TNF through TNFRs. This model would also explain why, when LTα3 is studied in settings where TNF is deleted, the dominant outcome is LTα3 acting as a pro-inflammatory cytokine45. Yet, further analysis using single-cell RNA sequencing (scRNA-seq) on immune cells that migrated from the intestine to the draining LNs in the presence or absence of anti-LTα3 revealed that, whereas outcomes demonstrating overt pathology such as weight loss were not evident when mice with WT immune cells were treated with anti-LTα3 mAb, neutralizing LTα3 still attenuated the appearance of activated follicular B cells, especially those that expressed Lta or Tnf. Quite remarkably, the appearance of Lta+ T cells was not evidently affected, such that anti-LTα3 seemed to especially target B cells, underscoring a similarity between genetic loss of the Lta gene in B cells and the impact of the anti-LTα3 mAb.
The loss of IgA+ plasma cells when B cells lacked Lta is reminiscent of a previous study wherein deficiency in LTβ versus LTα in ILCs differentially affected serum IgA46. Although it is possible that the loss of IgA+ plasma cells governs the factors that culminate in weight loss, luminal IgA itself is not the key factor in the systemic impact on weight observed in mice lacking Lta+ B cells, because loss of gut luminal IgA through epithelial Pigr deficiency led to heightened immune cell infiltration into the ileum, but weight loss did not ensue. Perhaps tissue IgA rather than luminal IgA is most relevant. Alternatively, the loss of IgA+ plasma cells may instead indicate the importance of LTα3 in facilitating passage of follicular B cells through the germinal center and differentiation process and that their critical functions are distinct from Ig secretion and perhaps include anti-inflammatory roles. We note that the potent anti-inflammatory mediator Il4i1 was highly expressed in B cells that depend on LTα3, but future work will be needed to sort out this mechanism.
Our findings could contribute to an understanding of why therapeutics such as etanercept that neutralize both LTα and TNF failed in IBD, whereas those selectively targeting TNF alone have shown significant efficacy47. One of the avenues of future investigation will be a search to better understand how LTα3 is linked to body weight. The link we uncovered in our studies, which we could not explain at the level of caloric intake or metabolic activity, is consistent with several other studies that have found possible regulation of body mass index or fat mass by polymorphisms or expression of LTα48,49,50. Although we were unable to detect failure to absorb nutrients, past studies have linked B cells to nutrient absorption29, so we cannot rule out subtle or micronutrient-selective effects on absorption. It seems that autologous LTα acts by influencing the fate of activated follicular B cells that go on to become ileal IgA+ plasma cells. Whereas these B cells might arise from the mLN, as we interrogated here, it is also possible that they arise in isolated lymphoid follicles of the small intestine that independently support the generation of IgA+ plasma cells29.
Beyond antibody production, LTα+ B cells may have other key anti-inflammatory roles in tissues. When these B cells are lost and inflammatory triggers are present, perhaps especially in the context of TNF-mediated inflammation, metabolic set points shift so fat catabolism ensues and, when fat stores are consumed, muscle catabolism begins. B cells have been linked extensively to metabolic dysfunction51 but not yet in the context studied here. These studies highlight the multifaceted roles of B cells in ileitis and raise several key directions that deserve more attention in the future.
Methods
Patient samples
Human mesentery samples were obtained from two different sources with written informed consent from each patient or their guardians. Discarded human mesentery tissues came from patients with CD that were undergoing bowel resections at Washington University School of Medicine. Control tissues came from surgical patients undergoing resection for gastrointestinal cancers or polyps. Staff affiliated with the Washington University Digestive Disease Research Core Center coordinated consent, oversaw tissue collection and managed de-identification services. Further control samples were obtained from organ donors consented for tissue use in research through Mid-America Transplant under a material transfer agreement. CD patient tissues were derived from inflamed or uninflamed regions of the ileum, collected under the supervision of a pathologist. Control ileal-draining mesentery was collected from patients with gastrointestinal cancer upstream of the tumor. Organ donors were excluded if they had previous bowel resections or a diagnosis of IBD. Participant demographics are summarized in Supplementary Table 1. Participants were not compensated. No statistical methods were used to predetermine sample sizes. All collections were conducted as approved by the Human Research Protection Office at Washington University (Institutional Review Board protocol no. 201111038) and followed the principles of the Declaration of Helsinki.
Mice
Mice were bred and housed at the Washington University vivarium in specific pathogen-free facilities under standard housing conditions of 12-h light/dark cycles. Food was provided ab libitum, cages were changed weekly and water was provided either through a Lixit valve or in water bottles that were changed twice a week. All experiments and procedures were approved by the Institutional Animal Care and Use Committee at Washington University (protocol nos. 20170154, 20-0032 and 22-0433). All mice were on a C57BL/6 background (Jackson Laboratory (JAX) cat. no. 000664); some were crossed to express the Ptprca CD45.1 allele (B6.SJL-Ptprca Pepcb/BoyJ, JAX cat. no. 002014). Other strains obtained from JAX were μMT (B6.129S2-Ighmtm1Cgn/J, JAX cat. no. 002288)52, CD19cre (B6.129P2(C)-Cd19tm1(cre)Cgn/J, JAX cat. no. 006785)53, Lta−/− (B6;129S2-Ltatm1Dch/J, JAX cat. no. 002257)54 and Tdtomato (B6.Cg-Gt(ROSA)26Sortm9(CAG-tdTomato)Hze/J, JAX cat. no. 007909) mice. Initial TNFΔARE breeder mice were provided through a material transfer agreement by the Cleveland Digestive Disease Research Core Center (NIH P30 DK097948). TNFΔARE were kept and bred as heterozygotes, cohoused with WT littermates. M. Baldridge (Washington University School of Medicine) provided Pigr−/− mice backcrossed to C57BL/6 mice (B6.129P2-Pigrtm1Fejo/Mmmh)55,56, and Ltb−/− (B6;129-Ltbtm1Flv/J)57 and TNFfl/fl mice58 were acquired from University of Texas Health San Antonio and expanded and used at Washington University after crossing to CD19Cre/+ mice. More detailed description of mouse breeding is given in the Supplementary Methods.
BM chimeras
Female mice (7–9 weeks old) were irradiated with 8 Gy in an X-ray irradiator, corresponding to a lethal dose without reconstitution, validated for our colony and irradiator. At 6 h after irradiation, mice received 5 million donor BM cells retro-orbitally, and were then housed for ≥8 weeks before further experiments.
Anti-LTα3, anti-TNF treatment and LTβR-Fc
Anti-LTα3 antibody S5H3 (S5H3 LT-a.Fc-MT)33 was a gift from Genentech33. Mice were weighed and then injected intraperitoneally with 6 mg kg−1 of body weight with anti-LTα3mAb or mouse IgG2a isotype control (Clone C1.18.4; Leinco Technologies) every 2 days. Some mice were also (or instead) injected with 600 μg anti-mouse TNF (clone TN3-19.12, Leinco Technologies)59 or Armenian Hamster IgG isotype control (clone PIP, Leinco Technologies). LTβR-Fc fusion protein60 and isotype control were provided by Biogen or Genentech33. These reagents were injected intraperitoneally weekly using 200 μg.
Human and mouse tissue and single-cell processing
Human ileal mesentery samples were collected in cold saline or RPMI-1640 (GIBCO, cat. no. 11875-085) and processed within 1 h. After rinsing in HBSS, the mesentery was separated using a scalpel, minced, weighed and digested (0.2 g tissue per milliliter) in RPMI-1640 with 10% fetal bovine serum (FBS) (ThermoFisher GIBCO, cat. no. 26140-079), 1 mg ml−1 Collagenase IV (Sigma-Aldrich, C5138) and 0.2 mg ml−1 DNase I (Sigma-Aldrich, cat. no. DN25) at 37 °C for 45 min, orbital shaking at 100 rpm. Samples were vortexed, filtered (100 μm) and washed in fluorescence-activated cell sorting (FACS) buffer (2% FBS, 5 mM EDTA (Corning, cat. no. 46-034-CI), 0.2% sodium azide in PBS) then centrifuged (10 min, 500g, 4 °C). Red blood cells (RBCs) were lyzed for 5 min, washed with 10 ml FACS buffer and spun again before resuspension in 1 ml FACS buffer. Cells were counted using an automatic cell counter (Cellometer Auto T4, Nexcelom), frozen at −80 °C in 10% DMSO/90% FBS then moved to liquid nitrogen. When needed, aliquots were thawed rapidly at 37 °C.
For collecting mouse mesentery, small intestine, MLNs, spleen, mice were perfused with cold PBS (pH 7.4) after blood plasma collection. For lamina propria (LP) isolations, intestines were cut longitudinally, washed in PBS to remove feces, then cut into 1-cm pieces. Mucus and epithelial cells were removed by incubation in HBSS (ThermoFisher, cat. no. 14175-079) containing 10% FBS, 20 mM HEPES (Corning, cat. no. 25-060-CI) and 5 mM EDTA, 20 min with shaking. Tissues were vortexed, gently agitated and revortexed. Murine mesentery and processed LP were then digested similarly to human tissues, described above. Leukocytes were enriched by centrifugation on a discontinuous Percoll gradient (70%/40%, GE Healthcare, cat. no. 17-0891-01) at 850g for 25 min at room temperature. Spleens and MLNs were crushed and passed through a 70-μm strainer; spleens underwent RBC lysis using BD Pharm Lyse (BD Biosciences, cat. no. 555899) for 5 min at RT. Blood was collected in EDTA-containing tubes, centrifuged (10,000g, 10 min, 4 °C) and plasma frozen. RBC were lyzed. Single-cell suspensions were resuspended in 1 ml FACS buffer and counted using the Cellometer Auto T4.
Flow cytometry
Single-cell suspensions were incubated with either murine FcBlock (BD Biosciences, cat. no. CD16/CD32, clone 2.4G2) or human FcR blocking reagent (Miltenyi Biotec, cat. no. 130-059-901) for 15 min at 4 °C, then stained with cell surface antibodies for 30 min at 4 °C. Dead cells were excluded using a Live/Dead Aqua Fixable Cell Stain Kit (Invitrogen, cat. no. L34957), 4′,6-diamidino-2-phenylindole (DAPI) or Helix NP Green (Biolegend, cat. no. 425303). Human cells were fixed before acquisition after Live/Dead Aqua Fixable Cell Stain by incubating the cells in 2% paraformaldehyde (PFA) for 15 min at 4 °C and then washed and resuspended. For ex vivo detection of TNF protein, cells were incubated for 4 h at 4 °C in Brefeldin-A (Biolegend, cat. no. 420601), before blocking, extracellular FACS staining, staining with Live/Dead Aqua Fixable Cell Stain, fixation and permeabilization (Biolegend, cat. no. 426803), and overnight staining with an anti-TNF antibody (TNF-PE; Biolegend, cat. no. MP6-XT22, 1:200)). For transcription factor staining, cells were fixed and permeabilized (eBioscience, cat. no. 00-5523-00) before blocking and staining for transcription factors. Human cells were stained for CD45-BUV395 (BD Biosciences, clone HI30, 1:100) or CD45-PerCP/Cyanine5.5 (Biolegend, clone H130, 1:100), CD19-PE or CD19-BV605 (Biolegend, clone HIB19, 1:200), and, as an exclusionary stain, CD3-Alexa Fluor 700 (Biolegend, clone HIT3a, 1:50), CD14-Alexa Fluor 700 (Biolegend, clone HCD14, 1:25) or CD16-Alexa Fluor 700 (Biolegend, clone 3G8, 1:50). Mouse cells were stained with cell surface antibodies in different combinations: CD45.1-Alexa Fluor 700 or CD45.1-PE-Cy7 (Biolegend, clone A20, 1:200), CD45.2-Pacific Blue, CD45.2-Alexa Fluor 700 or CD45.2-APC/Cy7 (Biolegend, clone 104, 1:200) or CD45.2-eFluor 506 (eBiosciences, clone 104, 1:200) or (Biolegend, clone 104, 1:200), pan-CD45-Pacific Blue or pan-CD45-BV421 (Biolegend, clone 30-F11, 1:400), CD4-BUV496 or CD4-BB700 (BD Biosciences, clone GK1.5, 1:400), CD8a-BV785 (Biolegend, clone 53-6.7, 1:400), CD11b-BUV737 (BD Biosciences, clone M1/70, 1:600), Ly6G-BUV805 (BD Biosciences, clone 1A8, 1:400), CD44-BV605 (Biolegend, clone IM7, 1:400), CD62L-PEDazzle 594 (Biolegend, clone MEL-14, 1:400), SiglecF-Alexa Fluor 647 (BD Biosciences, clone E50-2440, 1:200), TCRβ-PerCP/Cy5.5, TCRβ-BV711 or TCRβ-FITC (Biolegend, clone H57-597, 1:400), CD19-PE/Cy7 (Biolegend, clone 6D5, 1:400), CD11c-BUV395 (BD Biosciences, clone N418, 1:200), CD64-BV605 (Biolegend, clone X54-5/7.1, 1:200), CD115-BV605 (Biolegend, clone AFS98, 1:200), IgA-PE, IgA-FITC, or IgA-APC (eBioscience, clone mA-6E1, 1:200), MHC Class II-BUV496 (BD Bioscience, clone M5/114.15.2, 1:200) or MHC Class II-AF700 (Biolegend, clone M5/114.15.2, 1:200) and Ly6C (Biolegend, clone HK1.4, 1:200). Intercellular mouse staining used either TNF-PE (Biolegend, clone MP6-XT22, 1:200) or FOXP3-FITC (Invitrogen, clone FJK-16 s, 1:1,000), T-bet-eFluor 660 (Invitrogen, clone eBio4B10 (4B10), 1:400) and RORyt-BV421 (BD Biosciences, clone Q31-378, 1:800).
Cells were acquired on a LSR Fortessa X20, a FACSymphony, or a five-laser Cytek Aurora. Human cells were sorted for scRNA-seq on an Aria II in a Bioprotector cabinet. DAPI− CD45+ KikGR+ mouse cells were sorted for scRNA-seq on a Sony Synergy HAPS cell sorter.
Photoconversion of the ileum
TNFΔARE/+ or wild-type (WT) KikGR+ BM chimeras 30 weeks post-transplant were treated with anti-LTα3 mAb33 or isotype control before surgery to allow adequate systemic absorption, then anesthetized using inhaled isoflurane and placed on a heating pad. A midline incision exposed the intestines and a 365-nm fiber optic light source was used to photoconvert 15 discrete ileal regions, with each exposure for 10 s, then 30-s rest, three cycles per site. The peritoneum was closed with continuous absorbable 4-0 vicryl sutures and the skin closed with nonabsorbable nylon surgical knots. Mice recovered on a heating pad. A second dose of anti-LTα3 or isotype control (0.6 mg kg−1) was administered before tissue harvest. Mice were sacrificed in the order in which they were photoconverted. LNs were harvested, digested enzymatically as described above, and sorted on DAPI− CD45+ KikRED+ cells. Sorted cells were pooled from three mice for each experimental cohort.
Histology on distal ileum
The distal 2 cm of the ileum was measured, flushed with PBS, cut open, pinned on wax then fixed overnight in 4% PFA. Ileal tissue was washed in PBS, embedded in 2% agar and embedded for paraffin sectioning and staining with hematoxylin and eosin before scoring61.
Whole-mount imaging and confocal microscopy of the mouse mesentery
Intact mesenteries were pinned on wax, fixed with 4% PFA overnight at 4 °C, then washed in PBS, blocked and permeabilized in 5% donkey serum, 3% BSA, and 1% Triton X-100 in PBS buffer overnight at 4 °C. Tissue were incubated with antibodies to B220 (eBioscience, 14-0452-05), Lyve-1 (Abcam, ab14917), CSF1R (R&D, AF3818) diluted in 0.6% BSA and 0.2% Triton X-100 for at least 24 h, 4 °C. Samples were washed and incubated with donkey secondary antibody conjugated to Cy2, Cy3, or Cy5/Alexa Fluor 647 for at least 12 h, 4 °C. For quantification of TLSs, the tissue was blinded before proceeding. The mesentery was pinned flat on a SYLGARD® 184-coated (Ellsworth, 4019862) plate and dehydrated in ethanol, then cleared with methyl salicylate solution (Sigma-Aldrich, M6752) for10 min. For quantification of TLS, blinded mesenteries were examined using a Leica M205FA stereoscope while pinned to the SYLGARD plates. Mesenteries were quantified before unblinding. For confocal imaging, the mesenteries were instead cleared inside a custom-designed metal chamber with 0.2 mm thick coverslip glass bottom (Washington University Machine Shop) and imaged with the tissue remaining within the chamber, using a nine-laser inverted Leica SP8 microscope with full spectral hybrid detectors. Quantification of TLS area is described in the Supplementary Methods.
Whole-mount imaging of the mouse ileum or spleen using ADAPT-3D
Ileum (distal 2 cm) and spleen were rinsed in ice-cold PBS containing 10 U ml−1 heparin, fixed in 4% PFA (pH 9.0, 30% sucrose) for (ileum) or 24 h (spleen). Ileal segments were pinned mucosal side up before fixation. Fixed spleen was embedded in 4% agarose and sectioned at 200 µm on a vibratome. Tissues were processed for whole mount using the ADAPT-3D protocol62 (ADAPT-3D kit, Leinco Technologies, cat. no. A630). Briefly, ileal tissue was incubated for 24 h in ADAPT-3D Decolorization/Delipidation buffer62, washed twice for 30 min each in PBS, and blocked for 24 h in ADAPT-3D blocking buffer, then incubated for 24 h with unconjugated rabbit anti-mouse IgA (NSJ Bioreagents, cat. no. R20169) and mouse anti-mouse smooth-muscle actin–Cy3 (Sigma-Aldrich, cat. no. C6198). Samples were washed in PBS + 0.2% Tween-20 (PBST) and incubated for 24 h with donkey secondary antibody conjugated to Alexa Fluor 647. Spleen sections were blocked for 6–8 h in ADAPT-3D blocking buffer62, followed by a 6–8 h in Alexa Fluor 488-conjugated anti-mouse CD3ε (BioLegend, cat. no. 152322), Alexa Fluor 647 anti-mouse IgD (BioLegend, cat. no. 405708) and rat anti-mouse CD21/CD35 (BD Pharmingen, cat. no. 553817) conjugated to Mix-n-Stain CF568 (Biotium, cat. no. 92255). Sections were washed three times (1 h each) in PBST + 0.2% Tween-20, stained with DAPI for ≥2 h, equilibrated for 30 min in 0.5× and ≥ 2 h in 1× ADAPT-3D, mounted in fresh matching medium and imaged on a Leica TCS SP8 confocal microscope with a ×20/1.0 numerical aperture (NA) oil-immersion objective. Tile stitching and three-dimensional rendering used Imaris v.10.1.1 software.
Intravital lymphatic tracer imaging
Isoflurane-anesthestized mice were cut along the midline. Mesentery and intestine were splayed out onto a custom-built plate, kept moist and at physiological temperature with dripped, warm PBS at 37 °C (ref. 7). From 1 to 1.5 μl of 2,000 kDa fluorescein isothiocyanate (FITC)-Dextran (Sigma-Aldrich, cat. no. FD2000S or Invitrogen, cat. no. D7137) was injected into the most distal Peyer’s Patch of the ileum using a custom Hamilton syringe. Fluorescent signal (excitation, 488 nm; emission, 520 nm) along the exposed mesentery was recorded at 1 frame per second for 60 min (3,600 frames) or until the tracer reached the MLN7.
Metabolic cages and EchoMRI
Mice were weighed and body composition evaluated using an EchoMRI-100H 2n1 with a horizontal probe configuration (EchoMRI). Core body temperature was taken using rectal thermometer (RET-3 probe). Mice were housed singly in a 16-metabolic cage Comprehensive Laboratory Animal Monitoring System (CLAMS) (Columbus Instruments) and acclimated to cages for approximately 16 h, followed by 24 h of measurement occurring from 06:00 a.m. to 5:59 a.m. the next day at 22 °C, 12 h:12 h light:dark cycle, with cages positioned to distribute different experimental groups throughout in the CLAMS. Mice had ad libitum access to food and water, hung on load cells for measurement. Activity was monitored using infrared laser/detector arrays positioned at the level of the bedding on the x and y axes. Oxygen consumption and carbon dioxide production were measured by indirect calorimetry using a zirconia O2 sensor and CO2 sensor at air flow rates of 0.90 l min−1 (18-s line bleed, then a 2-s measurement per cage). Cages were measured individually in series, with CLAMS system enclosure air sampled each at 304-s intervals.
Assessment of intestinal permeability
Following a 4-h fast, mice received 167 mg kg−1 4-kDa FITC–dextran (Chondrex, cat. no. 4013) by gavage, then plasma was collected 2 h later for fluorescence intensity measurement (Cytation 5; excitation, 485 nm; emission, 525 nm). Relative permeability was calculated from the mean fluorescence intensity of the BMT cohort with WT B cells.
Enzyme-linked immunosorbent assay
Stool collection and processing are described in the Supplementary Methods. Stool homogenates or plasma were thawed on ice and assayed for Lipocalin-2/NGAL DuoSet ELISA kit (R&D Systems, cat. no. DY1857), or for IgA by sandwich enzyme-linked immunosorbent assay (ELISA) using high-binding 96-well plates (ThermoScientific, cat. no. 442404) coated overnight at 4 °C with rat anti-mouse IgA (SouthernBiotech, cat. no. 1165-01) at 2 µg ml−1 in carbonate–bicarbonate buffer (pH 9.2). The next day, wells were washed four times with PBS + 0.05% Tween-20, then plates were blocked with 1% BSA in PBS for 2 h at 22° (room temperature). Thawed plasma or stool was diluted in 1% BSA/PBS and incubated for 2 h at room temperature. After washing, detection used goat anti-mouse IgA-HRP (SouthernBiotech, cat. no. 1040-05) at 1:4,000 in 1% BSA/PBST at 2 h at room temperature. Tetramethylbenzidine substrate (Sigma; cat. no. T0440-100) was added at room temperature for 5–10 min in the dark, then stopped with 100 µl 1 N H2SO4 and absorbance read at 450 nm. IgA concentrations were interpolated from a four-parameter logistic fit of a standard curve generated with Mouse IgA-UNLB (SouthernBiotech, cat. no. 0106-01).
Assessment of ileal cytokines using Luminex
The distal 2 cm of ileum was collected, washed and snap-frozen until use. Thawed tissues were weighed, and lysates prepared using the M-PER Mammalian Protein Extraction Reagent (ThermoScientific, cat. no. 78501). Cytokine levels were assessed using the Milliplex Mouse Th17 Premixed 25 Plex Magnetic Bead (Millipore, cat. no. MT17MAG47K-PX25) were run on a FlexMAP 3D (Luminex) at the Bursky Center, Washington University. Cytokine levels were normalized to total protein in the lysate determined by the bicinchoninic acid assay.
Single-cell RNA profiling
After sorting, cells were placed on ice and centrifuged at 500g for 5 min, 4 °C. Supernatant was removed carefully and resuspended in 0.04% BSA in PBS at 1,000 cells μl−1. cDNA was prepared using gel beads in emulsion (GEM) generation and barcoding, followed by the GEM-reverse transcription (GEM-RT) reaction and bead cleanup steps. Purified cDNA was amplified for 11–16 cycles, then cleaned using solid phase reversible immobilization (SPRI) select beads, cDNA concentration was determined using a Bioanalyzer. For sample preparation on the 10x Genomics platform, the Chromium Next GEM Single Cell 5′ Kit v.2 was used for mouse cells and Chromium Single Cell 5′ Library Kit was used for human cells, along with the Chromium Next GEM Chip K Single Cell Kit, 48 reactions (cat. no. PN-1000286) and Dual Index Kit TT Set A. 96 reactions (cat. no. PN-1000215) were used. The concentration of each library was determined through quantitative PCR utilizing the KAPA library Quantification Kit (KAPA Biosystems/Roche) to produce cluster counts appropriate for the sequencer. For the mouse data, normalized libraries were sequenced on a NovaSeq X Plus Flow Cell, and the human single-cell data were sequenced across several Illumina NovaSeq 6000 high output runs. A median sequencing depth of 50,000 reads per cell was targeted for each Gene Expression library. Fastq files were generated using the Cell Ranger (10x Genomics) command mkfastq. For the human data, raw sequencing data were processed using the Cell Ranger multipipeline, v.7.1.0 (10x Genomics) using the human reference genome GRCh38-2020-A. For the mouse data, raw sequencing data was processed using the Cell Ranger multi pipeline, version 8.0.1 (10x Genomics) using the mouse reference genome mm10-2020-A to generate gene expression matrices. scRNA-seq data was processed using the R package Seurat63 (v.5.3.0). More detailed description of data processing is given in the Supplementary Methods.
Software
FlowJo (v.10.8.1, BD) was used for flow cytometry analysis. Linear regression was performed in R (v.4.4.3) using base functions and the model-based package (v.0.8.6); scatterplots were also made in R using ggplot2 (v.3.5.2), RColorBrewer (v.1.1-3), Seurat63 (5.3.0), scDblFinder64 (v.1.2.0) and patchwork (v.1.3.0). Other statistical analysis and graphing used GraphPad Prism (v.9.5.1 or v.10.4.2). Imaris (v.10.1.1) processed confocal and stereoscope images. Fiji software65 ((Fiji Is Just) ImageJ v.2.14.0/v.1.54f/Java v.1.8.0_322 (64-bit)) was used for TLS quantification. Photoshop Illustrator was used to assemble figures.
Statistical analysis and reproducibility
The number of patients or mice per experiment are stated in figure legends for all experiments. Distribution of the data was tested for normality in pilot experiments for each assay. For the types of data that were not normally distributed or had skewed population distributions across groups, nonparametric tests were used to test for significance. P < 0.05 was considered significant. Two-tailed Student’s t test, two-sided Mann–Whitney test, one-way ANOVA with post hoc Tukey test or Holm–Šídák test, two-way ANOVA with two-stage linear step-up method of Benjamini, Krieger and Yekutieli, and Kruskal–Wallis test with multiple comparisons corrected by Dunn’s test were used. For scRNA-seq data, testing for differentially expressed genes was calculated using the two-sided Wilcoxon rank-sum test, and P values were corrected using the Bonferroni method. Statistics were calculated using either GraphPad Prism (v.10.0.3) or R (v.4.4.3).
Each human patient or each mouse was considered one independent biological replicate. Blinding for the human patient data during processing and analysis was implausible, because physical tissue features made the phenotype obvious. Similarly, the weight loss and reduction in activity in mice with ileitis made blinding mice during most experiments impossible. However, histological scoring or counting the number of TLSs in the mesentery were done blinded, with unblinding done only after analysis was completed. For BMT experiments, mice were excluded from analyses if they had to be euthanized for predetermined reasons before the experimental endpoint, that is, if a BMT mouse lost ≥20% of their initial body weight, or if a mouse had insufficient reconstitution of from donor BM, defined as >5% of blood cells of recipient congenotype. Up to ten mice were irradiated simultaneously, whereas more than ten mice required irradiation in batches, in which case mice amongst groups were distributed evenly in the different irradiation rounds. For BMT donors, one donor was used for five recipients. Donor pools were made if more than five recipients of the same group were planned. Recipient mice in each experiment were obtained from Jackson Laboratories in the same shipment and assigned randomly to receive a given donor genotype. For neutralization experiments, mice were assigned randomly to receive a given treatment using Research Randomizer (http://www.randomizer.org/). All datapoints from all human patients are included. Pilot experiments used to optimize the experimental model, such as the length of time to wait for TLS development or work to optimize staining panels are not included in the final compiled data.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
De-identified human scRNA-seq data analyzed in this study are publicly available in the Gene Expression Omnibus (GEO) database (GSE255722). Mouse scRNA-seq data analyzed in this study are available at GEO GSE296503. Source data are provided with this paper.
Code availability
Customized code for both analysis of the scRNA-seq data and generation of some of the figures is available via GitHub at https://github.com/eerlich/B-cell-derived-lymphotoxins-in-TNF-mediated-ileitis.
References
Torres, J., Mehandru, S., Colombel, J. F. & Peyrin-Biroulet, L. Crohn’s disease. Lancet 389, 1741–1755 (2017).
Rogler, G., Singh, A., Kavanaugh, A. & Rubin, D. T. Extraintestinal manifestations of inflammatory bowel disease: current concepts, treatment, and implications for disease management. Gastroenterology 161, 1118–1132 (2021).
Crohn, B. B. & Janowitz, H. D. Reflections on regional ileitis, twenty years later. J. Am. Med. Assoc. 156, 1221–1225 (1954).
Warren, S. & Sommers, S. C. Pathology of regional ileitis and ulcerative colitis. J. Am. Med. Assoc. 154, 189–193 (1954).
Randolph, G. J. et al. Lymphoid aggregates remodel lymphatic collecting vessels that serve mesenteric lymph nodes in Crohn disease. Am. J. Pathol. 186, 3066–3073 (2016).
Rehal, S. & von der Weid, P. Y. TNFDeltaARE mice display abnormal lymphatics and develop tertiary lymphoid organs in the mesentery. Am. J. Pathol. 187, 798–807 (2017).
Czepielewski, R. S. et al. Ileitis-associated tertiary lymphoid organs arise at lymphatic valves and impede mesenteric lymph flow in response to tumor necrosis factor. Immunity 54, 2795–2811 (2021).
Rivera, E. D., Coffey, J. C., Walsh, D. & Ehrenpreis, E. D. The mesentery, systemic inflammation, and Crohn’s disease. Inflamm. Bowel Dis. 25, 226–234 (2019).
Kontoyiannis, D., Pasparakis, M., Pizarro, T. T., Cominelli, F. & Kollias, G. Impaired on/off regulation of TNF biosynthesis in mice lacking TNF AU-rich elements: implications for joint and gut-associated immunopathologies. Immunity 10, 387–398 (1999).
Roulis, M. et al. Host and microbiota interactions are critical for development of murine Crohn’s-like ileitis. Mucosal Immunol. 9, 787–797 (2016).
Schaubeck, M. et al. Dysbiotic gut microbiota causes transmissible Crohn’s disease-like ileitis independent of failure in antimicrobial defence. Gut 65, 225–237 (2016).
Fu, Y. X., Huang, G., Wang, Y. & Chaplin, D. D. B lymphocytes induce the formation of follicular dendritic cell clusters in a lymphotoxin alpha-dependent fashion. J. Exp. Med. 187, 1009–1018 (1998).
Tumanov, A. et al. Distinct role of surface lymphotoxin expressed by B cells in the organization of secondary lymphoid tissues. Immunity 17, 239–250 (2002).
Wang, Y. et al. Lymphotoxin beta receptor signaling in intestinal epithelial cells orchestrates innate immune responses against mucosal bacterial infection. Immunity 32, 403–413 (2010).
Andrews, J. S., Berger, A. E. & Ware, C. F. Characterization of the receptor for tumor necrosis factor (TNF) and lymphotoxin (LT) on human T lymphocytes. TNF and LT differ in their receptor binding properties and the induction of MHC class I proteins on a human CD4+ T cell hybridoma. J. Immunol. 144, 2582–2591 (1990).
Locksley, R. M. et al. Tumor necrosis factors alpha and beta differ in their capacities to generate interleukin 1 release from human endothelial cells. J. Immunol. 139, 1891–1895 (1987).
Broudy, V. C., Harlan, J. M. & Adamson, J. W. Disparate effects of tumor necrosis factor-alpha/cachectin and tumor necrosis factor-beta/lymphotoxin on hematopoietic growth factor production and neutrophil adhesion molecule expression by cultured human endothelial cells. J. Immunol. 138, 4298–4302 (1987).
Oster, W., Lindemann, A., Horn, S., Mertelsmann, R. & Herrmann, F. Tumor necrosis factor (TNF)-alpha but not TNF-beta induces secretion of colony stimulating factor for macrophages (CSF-1) by human monocytes. Blood 70, 1700–1703 (1987).
Murphy, M., Perussia, B. & Trinchieri, G. Effects of recombinant tumor necrosis factor, lymphotoxin, and immune interferon on proliferation and differentiation of enriched hematopoietic precursor cells. Exp. Hematol. 16, 131–138 (1988).
Browning, J. & Ribolini, A. Studies on the differing effects of tumor necrosis factor and lymphotoxin on the growth of several human tumor lines. J. Immunol. 143, 1859–1867 (1989).
Chicheportiche, Y. et al. TWEAK, a new secreted ligand in the tumor necrosis factor family that weakly induces apoptosis. J. Biol. Chem. 272, 32401–32410 (1997).
Castro-Dopico, T., Colombel, J. F. & Mehandru, S. Targeting B cells for inflammatory bowel disease treatment: back to the future. Curr. Opin. Pharmacol. 55, 90–98 (2020).
Lin, R. et al. Clinical significance of soluble immunoglobulins A and G and their coated bacteria in feces of patients with inflammatory bowel disease. J. Transl. Med 16, 359 (2018).
Palm, N. W. et al. Immunoglobulin A coating identifies colitogenic bacteria in inflammatory bowel disease. Cell 158, 1000–1010 (2014).
Harmsen, H. J., Pouwels, S. D., Funke, A., Bos, N. A. & Dijkstra, G. Crohn’s disease patients have more IgG-binding fecal bacteria than controls. Clin. Vaccin. Immunol. 19, 515–521 (2012).
Kontoyiannis, D. et al. Genetic dissection of the cellular pathways and signaling mechanisms in modeled tumor necrosis factor-induced Crohn’s-like inflammatory bowel disease. J. Exp. Med. 196, 1563–1574 (2002).
Chassaing, B. et al. Fecal lipocalin 2, a sensitive and broadly dynamic non-invasive biomarker for intestinal inflammation. PLoS ONE 7, e44328 (2012).
Franiak-Pietryga, I. et al. Activated B cells and plasma cells are resistant to radiation therapy. Int. J. Radiat. Oncol. Biol. Phys. 112, 514–528 (2022).
Gommerman, J. L., Rojas, O. L. & Fritz, J. H. Re-thinking the functions of IgA(+) plasma cells. Gut Microbes 5, 652–662 (2014).
Kumar, P. et al. Intestinal interleukin-17 receptor signaling mediates reciprocal control of the gut microbiota and autoimmune inflammation. Immunity 44, 659–671 (2016).
Ware, C. F. Network communications: lymphotoxins, LIGHT, and TNF. Annu. Rev. Immunol. 23, 787–819 (2005).
Muller, U., Jongeneel, C. V., Nedospasov, S. A., Lindahl, K. F. & Steinmetz, M. Tumour necrosis factor and lymphotoxin genes map close to H-2D in the mouse major histocompatibility complex. Nature 325, 265–267 (1987).
Chiang, E. Y. et al. Targeted depletion of lymphotoxin-alpha-expressing TH1 and TH17 cells inhibits autoimmune disease. Nat. Med. 15, 766–773 (2009).
Worm, M. & Geha, R. S. CD40 ligation induces lymphotoxin alpha gene expression in human B cells. Int Immunol. 6, 1883–1890 (1994).
Boussiotis, V. A., Nadler, L. M., Strominger, J. L. & Goldfeld, A. E. Tumor necrosis factor alpha is an autocrine growth factor for normal human B cells. Proc. Natl Acad. Sci. USA 91, 7007–7011 (1994).
Leon, B., Ballesteros-Tato, A., Misra, R. S., Wojciechowski, W. & Lund, F. E. Unraveling effector functions of B cells during infection: the hidden world beyond antibody production. Infect. Disord. Drug Targets 12, 213–221 (2012).
Aversa, G., Punnonen, J. & de Vries, J. E. The 26-kD transmembrane form of tumor necrosis factor alpha on activated CD4+ T cell clones provides a costimulatory signal for human B cell activation. J. Exp. Med. 177, 1575–1585 (1993).
Worm, M., Ebermayer, K. & Henz, B. Lymphotoxin-alpha is an important autocrine factor for CD40 + interleukin-4-mediated B-cell activation in normal and atopic donors. Immunology 94, 395–402 (1998).
Fritz, J. H. et al. Acquisition of a multifunctional IgA+ plasma cell phenotype in the gut. Nature 481, 199–203 (2011).
Tsutsui, H., Karasawa, S., Shimizu, H., Nukina, N. & Miyawaki, A. Semi-rational engineering of a coral fluorescent protein into an efficient highlighter. EMBO Rep. 6, 233–238 (2005).
Sadik, A. et al. IL4I1 is a metabolic immune checkpoint that activates the AHR and promotes tumor progression. Cell 182, 1252–1270 (2020).
Ashouri, J. F. & Weiss, A. Endogenous Nur77 is a specific indicator of antigen receptor signaling in human T and B cells. J. Immunol. 198, 657–668 (2017).
Levine, J. S. & Burakoff, R. Extraintestinal manifestations of inflammatory bowel disease. Gastroenterol. Hepatol. (NY) 7, 235–241 (2011).
Koeffler, H. P. et al. Recombinant human TNF alpha stimulates production of granulocyte colony-stimulating factor. Blood 70, 55–59 (1987).
Rusu, I. et al. Microbial signals, MyD88, and lymphotoxin drive TNF-independent intestinal epithelial tissue damage. J. Clin. Invest. 132, e154993 (2022).
Kruglov, A. A. et al. Nonredundant function of soluble LTalpha3 produced by innate lymphoid cells in intestinal homeostasis. Science 342, 1243–1246 (2013).
Ward-Kavanagh, L. K., Lin, W. W., Sedy, J. R. & Ware, C. F. The TNF receptor superfamily in co-stimulating and co-inhibitory responses. Immunity 44, 1005–1019 (2016).
Hamid, Y. H. et al. The common T60N polymorphism of the lymphotoxin-alpha gene is associated with type 2 diabetes and other phenotypes of the metabolic syndrome. Diabetologia 48, 445–451 (2005).
Pamir, N., McMillen, T. S., Edgel, K. A., Kim, F. & LeBoeuf, R. C. Deficiency of lymphotoxin-alpha does not exacerbate high-fat diet-induced obesity but does enhance inflammation in mice. Am. J. Physiol. Endocrinol. Metab. 302, E961–E971 (2012).
Upadhyay, V. & Fu, Y. X. Lymphotoxin signalling in immune homeostasis and the control of microorganisms. Nat. Rev. Immunol. 13, 270–279 (2013).
Camell, C. D. et al. Aging induces an Nlrp3 inflammasome-dependent expansion of adipose B cells that impairs metabolic homeostasis. Cell Metab. 30, 1024–1039 (2019).
Kitamura, D., Roes, J., Kuhn, R. & Rajewsky, K. A B cell-deficient mouse by targeted disruption of the membrane exon of the immunoglobulin mu chain gene. Nature 350, 423–426 (1991).
Rickert, R. C., Roes, J. & Rajewsky, K. B lymphocyte-specific, Cre-mediated mutagenesis in mice. Nucleic Acids Res. 25, 1317–1318 (1997).
De Togni, P. et al. Abnormal development of peripheral lymphoid organs in mice deficient in lymphotoxin. Science 264, 703–707 (1994).
Johansen, F. E. et al. Absence of epithelial immunoglobulin A transport, with increased mucosal leakiness, in polymeric immunoglobulin receptor/secretory component-deficient mice. J. Exp. Med. 190, 915–922 (1999).
Moon, C. et al. Vertically transmitted faecal IgA levels determine extra-chromosomal phenotypic variation. Nature 521, 90–93 (2015).
Alimzhanov, M. B. et al. Abnormal development of secondary lymphoid tissues in lymphotoxin beta-deficient mice. Proc. Natl Acad. Sci. USA 94, 9302–9307 (1997).
Grivennikov, S. I. et al. Distinct and nonredundant in vivo functions of TNF produced by t cells and macrophages/neutrophils: protective and deleterious effects. Immunity 22, 93–104 (2005).
Cope, A. P. et al. Chronic tumor necrosis factor alters T cell responses by attenuating T cell receptor signaling. J. Exp. Med. 185, 1573–1584 (1997).
Browning, J. L. et al. Characterization of lymphotoxin-alpha beta complexes on the surface of mouse lymphocytes. J. Immunol. 159, 3288–3298 (1997).
Erben, U. et al. A guide to histomorphological evaluation of intestinal inflammation in mouse models. Int J. Clin. Exp. Pathol. 7, 4557–4576 (2014).
Lee, D. D. et al. ADAPT-3D: accelerated deep adaptable processing of tissue for 3-dimensional fluorescence tissue imaging for research and clinical settings. Sci. Rep. https://doi.org/10.1038/s41598-025-16766-z (2025).
Hao, Y. et al. Dictionary learning for integrative, multimodal and scalable single-cell analysis. Nat. Biotechnol. 42, 293–304 (2024).
Germain, P. L., Lun, A., Garcia Meixide, C., Macnair, W. & Robinson, M. D. Doublet identification in single-cell sequencing data using scDblFinder. F1000Res 10, 979 (2021).
Schindelin, J. et al. Fiji: an open-source platform for biological-image analysis. Nat. Methods 9, 676–682 (2012).
Acknowledgements
We thank S. Bala and M. White (Department of Pathology, Washington University) and W. Spees (Department of Chemistry, Washington University) for technical expertise, A. Shaw (Genentech) for assistance with obtaining reagents and R. Brookheart, M. Baldridge and K. Choi for mentorship, training and guidance. We are grateful to A. Banks (Children’s Hospital, Harvard) for helpful discussions on metabolic studies. We also thank the Andrew M. and Jane M. Bursky Center for Human Immunology for assistance with Luminex cytokine assays, McDonnell Genome Sciences for support in scRNA sequencing, and the Alvin J. Siteman Cancer Center at Washington University School of Medicine and Barnes-Jewish Hospital in St. Louis, MO, for the use of the Siteman Flow Cytometry, which provided flow cytometry sorting service. This work was supported by NIH grants R01AI68044 and DP1DK130660 and grants from the Rainin Foundation to G.J.R. with additional support for the laboratory from NIH grants R37AI049653, U01AI163064, R01DK119147, DP1DK1130660 and P01AG078106. E.C.E. received support from T32AI007163 and T32DK077653, L.S.S. from T32 AR060719 and T32 AR007279. Q.A.A. was supported by the T32DK007130-52 Training program in academic gastroenterology, the Fellow to Faculty Transition Award (P25-01816) from the American Gastroenterological Association and a Dean’s Scholar Award from Washington University. R.S.C. received support from fellowship (FA-2020-01-IBD-1), the Lawrence C. Pakula, MD IBD Education and Innovation Fund and a Career Development Award from the Crohn’s and Colitis Foundation (grant no. 938100). A.K. received support from NIH grants T32AI007163 and T32AR007279, R.L.M. from NIH grant F30CA281124, J.H. from NIH grant K00CA264434, and H.S.R. from NIH grant T32DK007130. J.R.B. was supported by DP5028125 and the Burroughs Wellcome Fund (CAMS grant no. 1019648). A.V.T. was supported by Cancer Prevention and Research Institute of Texas (grant no. RP220470). Institutionally accessed support included use of core services funded by the Digestive Diseases Research Core Center at Washington University NIH P30 DK052574, Washington University Rheumatic Diseases Center NIH P30 AR073752, the Musculoskeletal Structure and Strength Core NIH P30 AR074992 and the Nutrition and Obesity Research Center NIH P30 DK056341. TNFΔARE mice were obtained from the Cleveland DDRCC, supported by NIH P30 DK097948. The Siteman Cancer Center is supported in part by an NCI Cancer Center Support grant no. P30 CA091842. E.C.E. is currently affiliated with Memorial Sloan Kettering Cancer Center, New York, NY; R.S.C. is affiliated with Augusta University, Augusta, GA. This work was conducted in partial fulfillment by E.C.E. of PhD thesis studies within the Immunology Program of the Roy and Diana Vagelos Division of Biological and Biomedical Sciences at Washington University.
Author information
Authors and Affiliations
Contributions
E.C.E. and G.J.R. conceived the study; E.C.E., R.S.C., J.R.B. and G.J.R. developed and organized study infrastructure; E.C.E., Q.A.A., A.K., R.L.M., C.G.H., J.H., H.S.R., R.L.F., T.J.D., L.S.S., R.S.C. and G.J.R. conducted experiments and analyzed data; E.C.E., F.G., J.R.B., R.S.C. and G.J.R. advised analyses; M.H.H., A.V.T., F.G., J.R.B. and G.J.R. provided key resources; G.J.R., F.G. and J.R.B. obtained regulatory compliance and funding; E.C.E., Q.A.A. and G.J.R. wrote the manuscript with editing input and additions from all authors.
Corresponding author
Ethics declarations
Competing interests
G.J.R. and R.S.C. receive royalties from the ADAPT-3D method for whole-mount imaging used in this study. The other authors declare no competing interests.
Peer review
Peer review information
Nature Immunology thanks Veronique Angeli, Yang-Xin Fu and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available. Primary Handling Editor: L. A. Dempsey, in collaboration with the Nature Immunology team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Quantification of cell types in mouse blood or regions of the small intestine.
a: Number of B cells in the blood by flow cytometry (four independent experiments, WT (n = 19), TNFΔARE/+ (n = 18), μMT-TNFΔARE/+ (n = 17)) b: Donor (CD45.2)+IgA+ plasma cells in the ileum by flow cytometry (three independent experiments, WT (n = 15), TNFΔARE/+ and μMT-TNFΔARE/+ (n = 14)). c: Percentages of FoxP3+RORγτ+ T regulatory cells. d: Number of neutrophils in the duodenum and jejunum by flow cytometry (2 independent experiments, WT and TNFΔARE/+ (n = 10) μMT-TNFΔARE/+ (n = 9)). e-g: Number of neutrophils (e) and monocytes (f) in the blood by flow cytometry (Four independent experiments, WT (n = 19), TNFΔARE/+ (n = 18) μMT-TNFΔARE/+ (n = 17)). g: Percentage of monocytes that are Ly6Chi in the blood (Three independent experiments, WT and TNFΔARE/+ (n = 14), μMT-TNFΔARE/+ (n = 12). Gating strategies for flow cytometry shown are in Supplementary Figs. 1–3. Kruskal-Wallis test on all with correction for multiple comparisons using Dunn’s test. All data depicted show mean ± SEM. Each symbol represents a single mouse.
Extended Data Fig. 2 B cells protect against weight loss in TNFΔARE/+ mice.
a-g: WT, TNFΔARE/+, and μMT-TNFΔARE/+ BM recipients 14 weeks post-transplant. a: Body weight of the mice at week 14. Mice were housed in metabolic cages for 24 h and scatterplots show energy expenditure (b), food intake (c), and water intake (d,e), quantified in the metabolic cages (d), and charted against body weight (e). Hydration ratio of the mice, measured by MRI, calculated as (total water - free water) / lean weight. f,g: Paws collected from WT, TNFΔARE/+, and μMT-TNFΔARE/+ BM recipients 16–17 weeks post-transplant showing the percentage of bone volume over total volume (f) and bone surface area over bone volume (g). (Three independent experiments WT and TNFΔARE/+ (n = 14), μMT-TNFΔARE/+ (n = 13)). For a, d-g data represent mean ± SEM. For b,c, lines and shaded areas represent a linear regression model fit per group. Each symbol represents a single mouse. One-way ANOVA with Tukey post-hoc test (a, e-g), Two-way ANOVA on d.
Extended Data Fig. 3 Impact of deleting Lta or Ltb in mixed bone marrow chimeras or reagents targeting LTbR or LTa3 on lymphoid tissue organization.
In Fig. 5, mixed BM chimeras were studied at 30–32 weeks after transplant, wherein WT recipient mice received μMT-TNFΔARE/+ BM at 90% and 10% marrow from WT, Lta−/− or Ltb−/− mice to resupply the B cell compartment with these respective genotypes. a: Vibratome sections of the spleen from these mice were made and immunostained for IgD, CD3, and CD21/CD35 to confirm that B cell deletions of Lta or Ltb altered splenic organization (Two independent experiments, n=2 spleens/group were imaged). b: Similar staining was made on spleen vibratome sections from TNFΔARE/+ mice that were treated with isotype control mAb, anti-LTα3 mAb, or LTβR-Ig for 2 days. Two independent experiments, n=2 spleens/group were imaged.
Extended Data Fig. 4 Analysis of the role of LTα in nutrient absorption.
a: Feces from a 24-h period of housing was collected from cages of mice that were recipients of WT/μMT-TNFΔARE/+ and Lta−/−/μMT-TNFΔARE/+ mixed bone marrow cells (cohort 1) or WT/μMT-TNFΔARE/+ and Ltb−/−/μMT-TNFΔARE/+ mixed bone marrow cells (cohort 2) to generate mice with ileitis with WT, LTα-deficient B cells (LTα−/− B cells), LTβ-deficient B cells (LTβ−/− B cells) with matched WT B cells compared in the respective cohorts. Feces from 4 to 5 mice in each cage were combined in each genotype of each cohort, dried and weighed, and subjected to bomb calorimetry. Data plot a combined value from each cage. b: TNFΔARE/+ mice were split into two cohorts and each was randomly assigned into groups (each 3 or 4 mice) that would receive anti-LTα3 mAb or isotype control. Fecal energy in the groups prior to administration of mAb versus the energy measured in feces over a 48-h treatment span (feces collected at 24 and 48 h in each cage) was assessed by bomb calorimetry. c,d: Plasma triglycerides were assessed after bolus gavage of olive oil in mice that were recipients of WT/μMT and Lta−/−/μMT mixed bone marrow cells (n=8 per group) (c) or in TNFΔARE/+ mice that were treated with anti-LTα3 or isotype control mAb for 2 days (n=4 per isotype, n=3 per anti-LTα3) (d). All mice in panels c and d were treated with poloxamer to inhibit lipolysis of triglycerides in the plasma during the assessment. Plasma measurements were performed as duplicates per mouse and timepoint and averaged together for a single value per mouse and timepoint. In c,d, data depicted show mean of the mouse groups ± SEM and each symbol represents a single time point per group.
Extended Data Fig. 5 Analysis of B cells and other CD45+ mesenteric cells by single cell RNAseq.
a: Graph on the left shows quantification of the number of CD19+ B cells in surgically resected sections of ileal-draining mesentery normalized to the weight of tissue (Control (n = 20), CD non-inflamed (n = 16), CD inflamed (n = 15)). Graph on the right depicts a subset of the data on left to show only CD patients that had matched mesentery draining from non-inflamed ileum and from inflamed ileum, lines connect paired samples from the same patient (n = 13). Gating strategy in Supplementary Fig. 4. Data depict mean ± SEM. Kruskal-Wallis test for graph on left, two-sided paired t test on for the graph on right. b, c: Single cell RNA sequencing (scRNA-seq) of sorted live, CD45+ mesenteric cells from 3 CD patients with matched mesentery draining from inflamed and non-inflamed regions of the ileum. Uniform Manifold Approximation and Projection [UMAP] plot of mesenteric immune cells showing if the cells are derived from inflamed or non-inflamed regions of the mesentery (b) and assigned cell classification of all the different UMAP clusters (c) shown in Fig. 7a. d: Heatmap of genes defining the clusters of CD human mesentery shown in c. e: Heatmap of genes defining the clusters of mouse mLN shown in Fig. 7c–e. f: A correlation scatter plot showing expression of Lta vs Tnf in mouse mLN in B cell cluster 1.
Extended Data Fig. 6 Assessment of the role of B cell-derived TNF in ileitis.
Mixed BM chimeras wherein WT recipient mice received μMT-TNFΔARE/+ BM at 90% and 10% marrow from CD19Cre/+ x Tdtomatofl/fl mice (n = 3) or CD19Cre/+ x TNFfl/fl x Tdtomatofl/fl mice (n = 5) were studied at 32 weeks after BMT to assess the role of B cell-derived TNF on ileitis. a: Flow cytometry gating strategy for TdTomato+ CD19+cells gated otherwise as in Supplementary Figs. 1 and 2. Flow cytometry on single cell suspensions of the distal ileum of these mice compared the two genotypes in impacting neutrophil accumulation (b) or accumulation of T cells (c) which were not statistically significantly different based on a two-sided Mann-Whitney test. d: TLSs were formed in the mesentery of the mice. Stereoscope micrography reveals Tdtomato+ follicles near the vascular and lymphatic tracts. Bright field images were overlaid on fluorescence so that the blood flowing in vessels also appears red and the surrounding mesenteric adipose tissue reflects light. e: Assessment of TLS face area in the two genotypes with measurements made of 26 TLSs from mice bearing WT B cells and measurements from 8 TLSs made from mice with Tnf−/− (TNF KO) B cells. The size of TLSs were not statistically significantly different based on a two-sided Mann-Whitney test to compare them. In b,c, each symbol represents a single mouse and data represent mean ± SEM.
Extended Data Fig. 7 A schematic summary figure.
This figure summarizes findings in this body of work. Figure created with BioRender.com.
Supplementary information
Supplementary Information
Supplementary Figs. 1–5 and Methods.
Supplementary Video 1
Lymphatic flow to the MLN in a mouse that received WT BM.
Supplementary Video 2
Blockage of lymphatic flow to the MLN in a mouse that received TNFΔARE/+ BM.
Supplementary Video 3
Lymphatic flow to the MLN in one TNFΔARE/+ chimeric mouse demonstrating a leak.
Supplementary Video 4
Restored lymphatic flow to the MLN in a mouse that received μMT-TNFΔARE/+ BM.
Supplementary Table 1
Excel sheet showing the age, sex and tissue type obtained from patients for human experiments.
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 5
Statistical source data.
Source Data Fig. 6
Statistical source data.
Source Data Fig. 7
Statistical source data.
Source Data Extended Data Fig. 1
Statistical source data.
Source Data Extended Data Fig. 2
Statistical source data.
Source Data Extended Data Fig. 4
Statistical source data.
Source Data Extended Data Fig. 5
Statistical source data.
Source Data Extended Data Fig. 6
Statistical source data.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Erlich, E.C., Alayo, Q.A., Kim, A. et al. Distinct roles for B cell-derived LTα3 and LTα1β2 in TNF-mediated ileitis. Nat Immunol 26, 1781–1793 (2025). https://doi.org/10.1038/s41590-025-02263-y
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41590-025-02263-y