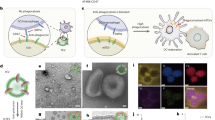
Abstract
Antigen-presenting cells phagocytose tumor cells and subsequently cross-present tumor-derived antigens. However, these processes are impeded by phagocytosis checkpoints and inefficient cytosolic transport of antigenic peptides from phagolysosomes. Here, using a microbial-inspired strategy, we engineered an antibody–toxin conjugate (ATC) that targets the ‘don’t eat me’ signal CD47 linked to the bacterial toxin listeriolysin O from the intracellular bacterium Listeria monocytogenes via a cleavable linker (CD47–LLO). CD47–LLO promotes cancer cell phagocytosis by macrophages followed by LLO release and activation to form pores on phagolysosomal membranes that enhance antigen cross-presentation of tumor-derived peptides and activate cytosolic immune sensors. CD47–LLO treatment in vivo significantly inhibited the growth of both localized and metastatic breast and melanoma tumors and improved animal survival as a monotherapy or in combination with checkpoint blockade. Together, these results demonstrate that designing ATCs to promote immune recognition of tumor cells represents a promising therapeutic strategy for treating multiple cancers.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
Data supporting the findings of this study are available within the article and its Supplementary Information. The sequencing data have been deposited in the National Center for Biotechnology Information Gene Expression Omnibus database under the publicly available accession number GSE255937. The GRCm39-based mouse reference genome is available from the UCSC Genome Browser (http://genome.ucsc.edu). Source data are provided with this paper.
References
Drago, J. Z., Modi, S. & Chandarlapaty, S. Unlocking the potential of antibody–drug conjugates for cancer therapy. Nat. Rev. Clin. Oncol. 18, 327–344 (2021).
Martiniova, L., Zielinski, R. J., Lin, M., DePalatis, L. & Ravizzini, G. C. The role of radiolabeled monoclonal antibodies in cancer imaging and ADC treatment. Cancer J. 28, 446–453 (2022).
Ackerman, S. E. et al. Immune-stimulating antibody conjugates elicit robust myeloid activation and durable antitumor immunity. Nat. Cancer 2, 18–33 (2021).
Shastry, M. et al. Rise of antibody–drug conjugates: the present and future. Am. Soc. Clin. Oncol. Educ. Book 43, e390094 (2023).
Motwani, M., Pesiridis, S. & Fitzgerald, K. A. DNA sensing by the cGAS–STING pathway in health and disease. Nat. Rev. Genet. 20, 657–674 (2019).
Jiang, M. et al. cGAS–STING, an important pathway in cancer immunotherapy. J. Hematol. Oncol. 13, 81 (2020).
Zhu, Y. et al. STING: a master regulator in the cancer–immunity cycle. Mol. Cancer 18, 152 (2019).
Amouzegar, A., Chelvanambi, M., Filderman, J. N., Storkus, W. J. & Luke, J. J. STING agonists as cancer therapeutics. Cancers 13, 2695 (2021).
Meric-Bernstam, F. et al. Phase I dose-escalation trial of MIW815 (ADU-S100), an intratumoral STING agonist, in patients with advanced/metastatic solid tumors or lymphomas. Clin. Cancer Res. 28, 677–688 (2022).
Harrington, K. J. et al. Preliminary results of the first-in-human (FIH) study of MK-1454, an agonist of stimulator of interferon genes (STING), as monotherapy or in combination with pembrolizumab (pembro) in patients with advanced solid tumors or lymphomas. Ann. Oncol. 29, 8, VIII712 (2018).
Janku, F. et al. Intratumoral injection of SYNB1891, a synthetic biotic designed to activate the innate immune system, demonstrates target engagement in humans including intratumoral STING activation. In Proceedings of the American Association for Cancer Research Annual Meeting CT110 (AACR, 2021).
Xu, M. M. et al. Dendritic cells but not macrophages sense tumor mitochondrial DNA for cross-priming through signal regulatory protein α signaling. Immunity 47, 363–373 (2017).
Lee, H. J., Woo, Y., Hahn, T. W., Jung, Y. M. & Jung, Y. J. Formation and maturation of the phagosome: a key mechanism in innate immunity against intracellular bacterial infection. Microorganisms 8, 1298 (2020).
Nguyen, B. N., Peterson, B. N. & Portnoy, D. A. Listeriolysin O: a phagosome-specific cytolysin revisited. Cell. Microbiol. 21, e12988 (2019).
Podobnik, M. et al. Plasticity of listeriolysin O pores and its regulation by pH and unique histidine [corrected]. Sci. Rep. 5, 9623 (2015).
Bavdek, A. et al. pH dependence of listeriolysin O aggregation and pore-forming ability. FEBS J. 279, 126–141 (2012).
Köster, S. et al. Crystal structure of listeriolysin O reveals molecular details of oligomerization and pore formation. Nat. Commun. 5, 3690 (2014).
Anand, P. K. et al. TLR2 and RIP2 pathways mediate autophagy of Listeria monocytogenes via extracellular signal-regulated kinase (ERK) activation. J. Biol. Chem. 286, 42981–42991 (2011).
Chávez-Arroyo, A. & Portnoy, D. A. Why is Listeria monocytogenes such a potent inducer of CD8+ T-cells? Cell. Microbiol. 22, e13175 (2020).
Hansen, K. et al. Listeria monocytogenes induces IFNβ expression through an IFI16-, cGAS- and STING-dependent pathway. EMBO J. 33, 1654–1666 (2014).
Chen, C. et al. The listeriolysin O PEST-like sequence co-opts AP-2-mediated endocytosis to prevent plasma membrane damage during Listeria infection. Cell Host Microbe 23, 786–795 (2018).
Repp, H. et al. Listeriolysin of Listeria monocytogenes forms Ca2+-permeable pores leading to intracellular Ca2+ oscillations. Cell. Microbiol. 4, 483–491 (2002).
Vadia, S. et al. The pore-forming toxin listeriolysin O mediates a novel entry pathway of L. monocytogenes into human hepatocytes. PLoS Pathog. 7, e1002356 (2011).
Xiao, L., Wang, Q. & Peng, H. Tumor-associated macrophages: new insights on their metabolic regulation and their influence in cancer immunotherapy. Front. Immunol. 14, 1157291 (2023).
Plaza-Ga, I. et al. pH-triggered endosomal escape of pore-forming listeriolysin O toxin-coated gold nanoparticles. J. Nanobiotechnology 17, 108 (2019).
Mulvihill, E., van Pee, K., Mari, S. A., Müller, D. J. & Yildiz, Ö. Directly observing the lipid-dependent self-assembly and pore-forming mechanism of the cytolytic toxin listeriolysin O. Nano Lett. 15, 6965–6973 (2015).
Dufour, A. et al. Automated quantification of cell endocytosis using active contours and wavelets. In 2008 19th International Conference on Pattern Recognition (IEEE, 2008).
de Chaumont, F. et al. Icy: an open bioimage informatics platform for extended reproducible research. Nat. Methods 9, 690–696 (2012).
Dersh, D., Yewdell, J. W. & Wei, J. A SIINFEKL-based system to measure MHC class I antigen presentation efficiency and kinetics. Methods Mol. Biol. 1988, 109–122 (2019).
Porgador, A., Yewdell, J. W., Deng, Y., Bennink, J. R. & Germain, R. N. Localization, quantitation, and in situ detection of specific peptide–MHC class I complexes using a monoclonal antibody. Immunity 6, 715–726 (1997).
Jenkins, M. H. et al. Multiple murine BRafV600E melanoma cell lines with sensitivity to PLX4032. Pigment Cell Melanoma Res. 27, 495–501 (2014).
Abdelfattah, N. et al. Single-cell analysis of human glioma and immune cells identifies S100A4 as an immunotherapy target. Nat. Commun. 13, 767 (2022).
Benguigui, M. et al. Interferon-stimulated neutrophils as a predictor of immunotherapy response. Cancer Cell 42, 253–265 (2023).
Gungabeesoon, J. A neutrophil response linked to tumor control in immunotherapy. Cell 186, 1448–1464 (2023).
Hirschhorn, D. et al. T cell immunotherapies engage neutrophils to eliminate tumor antigen escape variants. Cell 186, 1432–1447 (2023).
Ma, R. Y. et al. Macrophage diversity in cancer revisited in the era of single-cell omics. Trends Immunol. 43, 546–563 (2022).
Stables, M. J. et al. Transcriptomic analyses of murine resolution-phase macrophages. Blood 118, 192–208 (2011).
Espinosa-Carrasco, G. et al. Intratumoral immune triads are required for immunotherapy-mediated elimination of solid tumors. Cancer Cell 42, 1202–1216 (2024).
Magen, A. et al. Intratumoral dendritic cell–CD4+ T helper cell niches enable CD8+ T cell differentiation following PD-1 blockade in hepatocellular carcinoma. Nat. Med. 29, 1389–1399 (2023).
Andreatta, M. et al. Interpretation of T cell states from single-cell transcriptomics data using reference atlases. Nat. Commun. 12, 2965 (2021).
Jin, S. et al. Inference and analysis of cell–cell communication using CellChat. Nat. Commun. 12, 1088 (2021).
Alvarez-Martinez, M. et al. Blimp-1 and c-Maf regulate immune gene networks to protect against distinct pathways of pathobiont-induced colitis. Nat. Immunol. 25, 886–901 (2024).
McCarthy, E. F. The toxins of William B. Coley and the treatment of bone and soft-tissue sarcomas. Iowa Orthop. J. 26, 154–158 (2006).
Mason, N. J. et al. Immunotherapy with a HER2-targeting Listeria induces HER2-specific immunity and demonstrates potential therapeutic effects in a phase I trial in canine osteosarcoma. Clin. Cancer Res. 22, 4380–4390 (2016).
Neeson, P., Pan, Z. K. & Paterson, Y. Listeriolysin O is an improved protein carrier for lymphoma immunoglobulin idiotype and provides systemic protection against 38C13 lymphoma. Cancer Immunol. Immunother. 57, 493–505 (2008).
Paterson, Y., Guirnalda, P. D. & Wood, L. M. Listeria and Salmonella bacterial vectors of tumor-associated antigens for cancer immunotherapy. Semin. Immunol. 22, 183–189 (2010).
Sewell, D. A. et al. Recombinant Listeria vaccines containing PEST sequences are potent immune adjuvants for the tumor-associated antigen human papillomavirus-16 E7. Cancer Res. 64, 8821–8825 (2004).
Singh, R., Dominiecki, M. E., Jaffee, E. M. & Paterson, Y. Fusion to listeriolysin O and delivery by Listeria monocytogenes enhances the immunogenicity of HER-2/neu and reveals subdominant epitopes in the FVB/N mouse. J. Immunol. 175, 3663–3673 (2005).
Witte, C. E. et al. Innate immune pathways triggered by Listeria monocytogenes and their role in the induction of cell-mediated immunity. Adv. Immunol. 113, 135–156 (2012).
Li, B. T. et al. A phase 1/2 study of a first-in-human immune-stimulating antibody conjugate (ISAC) BDC-1001 in patients with advanced HER2-expressing solid tumors. J. Clin. Oncol. 41, 2538 (2023).
US FDA. NDA/BLA Multi-Disciplinary Review and Evaluation BLA 761104, Lumoxiti, Moxetumomab Pasudotox www.accessdata.fda.gov/drugsatfda_docs/nda/2018/761104Orig1s000MultidisciplineR.pdf (2018).
Janku, F. et al. Preclinical characterization and phase I study of an anti-HER2–TLR7 immune-stimulator antibody conjugate in patients with HER2+ malignancies. Cancer Immunol. Res. 10, 1441–1461 (2022).
Chang, H.-P. et al. Pharmacokinetics and pharmacodynamics of antibody–drug conjugates administered via subcutaneous and intratumoral routes. Pharmaceutics 15, 1132 (2023).
Osorio, J. C. et al. The antitumor activities of anti-CD47 antibodies require Fc–FcγR interactions. Cancer Cell 11, 2051–2065 (2023).
Bradley, C. A. CD24 — a novel ‘don’t eat me’ signal. Nat. Rev. Cancer 19, 541 (2019).
Lu, Y. et al. Immunological conversion of solid tumours using a bispecific nanobioconjugate for cancer immunotherapy. Nat. Nanotechnol. 17, 1332–1341 (2022).
Li, X. et al. Cancer immunotherapy based on image-guided STING activation by nucleotide nanocomplex-decorated ultrasound microbubbles. Nat. Nanotechnol. 17, 891–899 (2022).
Acknowledgements
We thank K. Duprez and F. Han (Structure Based Design, Inc.) and Bio-Synthesis, Inc. for expertise with protein–antibody conjugation, K. Dunner, Jr (High Resolution Electron Microscopy Facility) for help with transmission electron microscopy image acquisition, D. Fisher (Massachusetts General Hospital) for the gift of the D4M.3A melanoma cell line, J. Zhang of MD Anderson’s Department of Experimental Radiation Oncology for processing histologic samples; V. Van and K. L. Maldonado at MD Anderson’s Small Animal Imaging Facility for helping with the animal experiments, N. R. Vaughn and N. Nguyen at MD Anderson’s Flow Cytometry and Cellular Imaging Core Facility for helping with flow cytometry experiments, C. Shi and J. Yan at MD Anderson’s Oncology Research for Biologics and Immunotherapy Translation platform for helping with ADA experiments and C. Wogan from MD Anderson’s Division of Radiation Oncology for editorial help. This work was supported in part by the National Institutes of Health (R01NS117828 to W.J.) and the American Cancer Society (RSG-22-052-01-IBCD to W.J.), the Radiological Society of North America Resident Grant (to B.R.S.), the SITC Merck Cancer Immunotherapy Clinical Fellowship (to B.R.S.), the American Society of Clinical Oncology Young Investigator Award (to B.R.S.), the American Cancer Society award (PF-24-1156745-01-ET, grant https://doi.org/10.53354/ACS.PF-24-1156745-01-ET.pc.gr.193703 (to B.R.S.)), the Susan G. Komen Foundation Career Transition Award (to Y.W.) and the ABTA and Uncle Kory Foundation Fellowship (to K.H.).
Author information
Authors and Affiliations
Contributions
W.J., B.Y.S.K., B.R.S. and Y.W. conceived the project and were responsible for all phases of the research. B.R.S. conducted the majority of the experiments and data analyses. Y.W., A.W., N.T., D.L., J.E., K.H., S.D., J.H., Y.M., A.G., S.D.J., M.C., M.K., T.D.G., A.C.K., J.L. and A.A. assisted with data collection and interpretation. N.T. and K.Y. performed bioinformatics analysis. The manuscript was drafted by B.R.S., B.Y.S.K. and W.J. and was revised and approved by all authors.
Corresponding authors
Ethics declarations
Competing interests
A provisional patent application based on the technology described in the paper has been filed by the Board of Regents, the University of Texas System, with W.J., B.R.S., Y.W. and B.Y.S.K. as inventors. The other authors declare no competing interests.
Peer review
Peer review information
Nature Cancer thanks Dennis Discher and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Conjugation of anti-CD47 and IgG1 to Listeriolysin O.
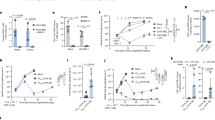
a, Results of sheep red blood cell (sRBC) haemolysis assay of purified LLO. Data show n = 3 biologically independent experiments. b, The MIAP410 antibody (BioXCell, Cat#BE0283) or the IgG1 antibody (BioXCell, Cat#BE0083) was dialyzed against phosphate-buffered saline (PBS) buffer, pH 7.2, and the dialyzed antibody was then modified with the click chemistry labelling reagent DBCO-PEG4-NHS ester (Conju-Probe, SKU# CP-2028) for 30 min at room temperature. The crosslinker in excess was removed by dialysis against PBS buffer, pH 7.2. c, The buffer of Listeriolysin O protein (Genscript) was exchanged with PBS buffer, pH 7.2, by PD-10 column (GE healthcare, Cat# 17085101), followed by modification with the click chemistry crosslinking reagent SPDP-PEG11-azide (BroadPharm, Cat# BP-25143). d, SDS-PAGE results of IgG-LLO conjugate in reductive and non-reductive loading buffers. Representative gel of n = 4 biological replicates shown.
Extended Data Fig. 2 In vitro toxicity assays of CD47-LLO with cancer cell lines.
a-h, Flow cytometry analysis and quantification of apoptosis and necrosis in 4T1Br4 (a-b), EO771 (c-d), KPC (e-f), and D4M.3 A (g-h) cells after treatment with 2 μg/mL CD47-LLO for 0, 6, or 24 hours (n = 4 biologically independent experiments for b, d, f, and n = 3 for h). Data shown represent mean ± s.d. (b, d, f, h) analyzed by one-way analysis of variance with Tukey’s multiple comparisons test.
Extended Data Fig. 3 CD47-LLO promotes dendritic cell phagocytosis, lysosomal permeabilization, antigen presentation, and cGAS-STING activation in vitro.
a, Flow cytometry analysis and b, quantification of phagocytic activity of bone marrow-derived dendritic cells (BMDCs) as evaluated by flow cytometry. BMDCs were collected from n = 3 C57BL6 mice. c, Flow cytometry analysis and d, quantification of BMDCs stained with acridine orange. BMDCs were collected from n = 4 C57BL6 mice for IgG treatment and n = 3 for remaining treatment groups. e, Flow cytometry analysis and f, quantification of cross-presentation of SIINFEKL–H2Kb peptides on the surfaces of BMDCs (n = 3). g, Flow cytometry analysis and h, quantification of pSTING levels in BMDCs isolated from co-cultures with EO771 cells. n = 4 C57BL6 mice. Data shown represent mean ± s.d. (b, d, f, h) analyzed by one-way analysis of variance with Tukey’s multiple comparisons test.
Extended Data Fig. 4 Antitumour effect of intratumoural CD47-LLO in 4T1Br4 and EO771 models.
a, Schematic of experiments involving intratumoural (IT) injections of CD47-LLO or anti-CD47 in syngeneic orthotopic 4T1Br4 breast cancer models. b, 4T1Br4 tumour volumes after IT injection of anti-CD47 or CD47-LLO. n = 4 for intratumoural CD47-LLO, n = 4 for intratumoural anti-CD47. c-d, Flow cytometry analysis of CD4+ (c) and CD8+ (d) tumour-associated lymphocytes in EO771 tumours at day 16 after tumour inoculation in each group. e, Flow cytometry analysis of SIINFEKL–H2Kb tetramer+CD8+ T cells within the tumour microenvironment. Data shown represent mean ± s.e.m. (b) analyzed by two-way analysis of variance with Tukey’s multiple comparisons test. Panel a created by modifying graphics from BioRender.com.
Extended Data Fig. 5 Biodistribution of CD47-LLO after intratumoural and intraperitoneal administration in vivo.
a, Representative fluorescence images and b, quantification of EO771 tumour-bearing mice taken at predetermined times after intratumoural injection of IR800CW-tagged CD47-LLO (25 μg). n = 2 mice. Bilateral tumours enclosed in circles. c, Ex vivo fluorescence images and d, quantification of tumour and major organs collected at 24 h after intratumoural administration. n = 2 mice. e, Quantification of EO771 tumours taken at predetermined times after intraperitoneal injection of IR800CW-tagged CD47-LLO (100 μg). n = 4 mice. f, Ex vivo fluorescence images and g, quantification of tumour and major organs collected at 36 h after intraperitoneal administration. n = 4 mice. Data shown represent mean ± s.d. (b, d, e, g) analyzed by two-sided unpaired Student’s t test (g).
Extended Data Fig. 6 Tumour-associated CD45+ cells detected by single-cell RNA sequencing.
a, Uniform manifold approximation and projection (UMAP) of all single cells from 3 treatment groups color-coded by cluster from IgG (n = 3), anti-CD47 (n = 4), or CD47-LLO (n = 4) treated tumours. b, Heatmap of 20 differentially expressed genes in clusters, ranked by false discovery rate (FDR) from the 3 treatment groups. c, Dot plot showing marker expression for different clusters from the 3 treatment groups. Dot size indicates the percentage of cells in each cluster expressing the gene and colors indicate the average expression levels.
Extended Data Fig. 7 Tumour-associated neutrophils are enriched in CD47-LLO tumours.
a, Representative images show levels of Ly-6g+ (top) and Ly-6g + /Cd11b+ (bottom) cells detected by immunostaining in 4T1Br4 breast tumour frozen tissue sections. Scale bar, 10 μm. b, Quantification of Ly-6g+ cells per DAPI+ cells per field of view (n = 15 per treatment condition from n = 3 biologically independent tumours per condition). c, Uniform manifold approximation and projection (UMAP) of only CD45+ granulocytes color-coded by sample from IgG (n = 3), anti-CD47 (n = 4), or CD47-LLO (n = 4) treated tumours. d, UMAP projection of only CD45+ granulocytes color-coded by cluster. e, Numbers of cells (y-axis) from each cluster (x-axis) color-coded by sample. f, Percentage of cells (y-axis) from each cluster (x-axis) color-coded by sample. g, Dot plot depicting the top 5 differentially expressed genes per granulocyte cluster. The dot size indicates the percentage of cells in each cluster expressing the gene and colors indicate the average expression levels. Data shown represent mean ± s.d. (b) analyzed by two-sided unpaired Student’s t test.
Extended Data Fig. 8 Differentially expressed genes define tumour-associated macrophages clusters.
a, Dot plot depicting the top 10 differentially expressed genes per macrophage cluster. Dot size indicates the percentage of cells in each cluster expressing the gene and colors indicate the average expression levels from IgG (n = 3), anti-CD47 (n = 4), or CD47-LLO (n = 4) treated tumours. b, Dot plot depicting the differential expression of relevant genes for cluster assignments per macrophage cluster from the 3 treatment groups. c, Gene set enrichment analysis utilizing the Gene Ontology Biological Process (GOBP) gene set for tumour-associated macrophages with heatmap displaying the ten most upregulated and downregulated pathways in each cluster ranked by their normalized enrichment scores (NES) from the three treatment groups.
Extended Data Fig. 9 Tumour-infiltrating CD8 T cells show decreased PD-1 positivity with CD47-LLO and anti-PD-1 treatment.
a, Schema for generating in vivo syngeneic orthotopic breast cancer models for intraperitoneal injection of CD47-LLO, anti-CD47, and anti-PD1. b, Flow cytometry analysis and c, quantification of PD-1 negativity in CD8 T cells isolated from 4T1Br4 tumours treated with anti-CD47, CD47-LLO, anti-CD47 + anti-PD-1, or CD47-LLO + anti-PD-1. Data show mean ± s.d. (c) analyzed by one-way analysis of variance with Tukey’s multiple comparisons test. Data show n = 4 mice per treatment group.
Extended Data Fig. 10 In vivo toxicity of CD47-LLO.
a-b, Lymphocyte (a) and red blood cell (b) counts at day 2 and day 9 after intraperitoneal injection of drug (50 μg IgG, 50 μg anti-CD47, 100 μg CD47-LLO, or 100 μg CD47-LLO + 200 μg anti-PD1). Data show n = 4 mice for IgG and anti-CD47 treatment groups and n = 3 for other treatment groups. c, Serum blood urea nitrogen (BUN) levels and d, serum aspartate transaminase / alanine transaminase (AST/ALT) levels at day 9 after drug injection. Data show n = 4 mice for IgG and anti-CD47 treatment groups and n = 3 for other treatment groups. e, Body weight changes in mice at day 2 and day 9 after drug injection. Data show n = 4 mice for IgG and anti-CD47 treatment groups and n = 3 for other treatment groups. f, Hematoxylin and eosin staining of paraffin sections of major organs two days after intraperitoneal injection of CD47-LLO or CD47-LLO and anti-PD-1. Experiment was repeated independently n = 3 times with similar results; a representative result is shown. Scale bar, 200 mm. g, Serum IL-6 levels at 4 hours after drug injection measured by enzyme-linked immunosorbent assay (ELISA). Data show n = 4 C57BL6 mice per treatment group. h, Serum IL-1β levels at 4 hours after drug injection measured by ELISA. Data show n = 4 C57BL6 mice per treatment group. i, Serum concentrations of anti-LLO antibody at 6 weeks after drug injection by sandwich immunogenicity assay. Data show n = 3 mice per IgG treatment group and n = 5 mice per CD47-LLO treatment group. Data shown represent mean ± s.d. (a, b, c, d, e, g, h, i) analyzed by one-way analysis of variance with Tukey’s multiple comparisons test (a, b, c, d, e) or two-sided unpaired Student’s t test (g, h, i).
Supplementary information
Supplementary Information
Supplementary Figs. 1–5 and Table 1.
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 5
Statistical source data.
Source Data Fig. 6
Statistical source data.
Source Data Fig. 7
Statistical source data.
Source Data Fig. 8
Statistical source data.
Source Data Extended Data Fig. 1
Statistical source data.
Source Data Extended Data Fig. 2
Statistical source data.
Source Data Extended Data Fig. 3
Statistical source data.
Source Data Extended Data Fig. 4
Statistical source data.
Source Data Extended Data Fig. 5
Statistical source data.
Source Data Extended Data Fig. 7
Statistical source data.
Source Data Extended Data Fig. 9
Statistical source data.
Source Data Extended Data Fig. 10
Statistical source data.
Source Data Figs. 1 and 3 and Extended Data Fig. 1
Uncropped blots and gels.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Schrank, B.R., Wang, Y., Wu, A. et al. An antibody–toxin conjugate targeting CD47 linked to the bacterial toxin listeriolysin O for cancer immunotherapy. Nat Cancer 6, 511–527 (2025). https://doi.org/10.1038/s43018-025-00919-0
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s43018-025-00919-0