Abstract
Galanin, a neuropeptide, regulates immune and inflammatory responses via GALR1-3. GALRs have emerged as potential therapeutic targets for inflammatory bowel disease (IBD), yet their mechanistic roles remain unclear. Based on evolutionary analysis, we identified a long galanin isoform (GAL53), generated by alternative splicing in non-mammalian vertebrates. Here we show that the chicken ortholog cGAL53 is robustly expressed in colonic tissue but downregulated upon dextran sulfate sodium (DSS)-induced colitis. Administration of cGAL53 alleviates colitis-associated weight loss, colon shortening, bleeding, and inflammation in both chickens and mice. These effects are abolished in Galr2-deficient mice, highlighting receptor dependency. Moreover, epithelial cell-specific Arrb2 and Gnaq knockout models demonstrate that cGAL53 protects the gut barrier and reduces inflammation by activating β-arrestin2-biased GALR2 signaling. Our findings reveal a naturally occurring long galanin peptide with potent anti-inflammatory activity and propose evolutionary medicine-guided biased GALR2 agonism as a therapeutic strategy for IBD.
Similar content being viewed by others
Introduction
Inflammatory bowel disease (IBD) is a chronic inflammatory condition of the gastrointestinal tract with two primary forms: Crohn’s disease (CD) and ulcerative colitis (UC)1. In addition to inflammation, these conditions are further characterized by damage to the lining of the digestive system, leading to symptoms, such as abdominal pain, diarrhea, fatigue, bloody stools, and weight loss2,3. Although the exact cause of IBD remains unclear, it is suspected to involve a combination of genetic predisposition, immune system dysfunction, environmental factors, and an imbalance in the gut microbiome3,4,5. Over the past few decades, the incidence of IBD has been increasing worldwide. Given that IBD remains incurable and can even be mortal, managing it imposes significant direct and indirect healthcare burdens6. Therefore, there is an urgent need for effective treatment strategies to mitigate the increasing prevalence of IBD.
In mammals, galanin, as a biologically active neuropeptide, is composed of 29/30 amino acids (29 in rodents, 30 in primates) and is widely expressed in the central nervous system, endocrine system, and the enteric nervous system components of the gastrointestinal tract7. It is derived from a precursor consisting of 123 amino acids, known as preprogalanin (ppGAL), which contains a signal peptide, the mature galanin peptide, as well as a 59-amino-acid galanin-message-associated peptide (GMAP)8. Galanin is evolutionarily conserved, with its amino acid sequences showing high similarity across different species (Fig. 1a and Supplementary Fig. 1b, c). This conservation is especially pronounced at the N-terminal region, which plays a crucial role in agonist-receptor binding9,10. Galanin interacts with its receptors (GALRs) that belong to the G-protein-coupled receptor (GPCR) superfamily comprising three subfamilies, namely GALR1, GALR2, and GALR3 in mammals, featuring high sequence and structural similarity11. Nevertheless, their tissue distribution patterns and the specific G proteins they activate for signal transduction vary12. GALR1 is expressed in the basal forebrain, hypothalamus, and spinal cord, as well as the small intestine and heart, whereas GALR2 is widely distributed in the brain, pituitary gland, and peripheral tissues13,14,15,16. GALR3 is expressed at moderate levels only in discrete brain regions and at low levels in many central and peripheral tissues17,18. GALR1 and GALR3 predominantly couple to Gi/o protein and inhibit adenylyl cyclase to decrease the cAMP levels, while GALR2 can activate G12/13, Gq/11, and β-arrestin1/2 pathway in addition to Gi/o11,19,20. Galanin exhibits different affinities for the three receptors, with high affinity for GALR1 and GALR2, and lower affinity for GALR321. The diversity of receptors and signaling pathways endows galanin with a broad range of physiological functions, such as regulating circadian rhythms, brain development, pain, food intake, stress, metabolism, and inflammation9,22.
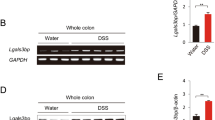
a Galanin long isoform is present in bony fish, amphibians, reptiles, and birds, and the gene locus is conserved. The red box represents the inserted amino acids. b The insertion within the long galanin isoform is due to an additional exon (ex. 3a). c Sequence alignment of the mature galanin peptide across vertebrate species. The expression of chicken Galanin (d) in jejunum and its receptors (e) in jejunum and ileum in the control and DSS treatment group. Each data point represents one chicken (n = 8). Data are presented as mean ± SD, analyzed by two-sided Student’s t test. Statistically significant differences between the control and DSS groups are indicated with asterisks: *p < 0.05. f Experimental scheme of DSS-induced intestinal inflammation in chickens initially 21 days of age. g Body weight changes throughout the experimental period (n = 7/group). Data are presented as mean ± SD, analyzed using two-way ANOVA. Statistically significant are indicated with asterisks: ***p < 0.001; ****p < 0.0001. Representative images of H&E staining and histopathology-associated index (HAI) analysis of jejunum (h, i) and ileum (j, k) (n = 3/group). Scale bar = 100 μm. ELISA detected cytokine (IL-6, TNF-α, and IL-1β) levels in chicken jejunum (l) and ileum (m) homogenates. Sample size for each group is as follows: jejunum: control (n = 10), DSS (n = 10), cGAL29 (n = 10), cGAL53 (n = 11); Ileum: control (n = 9), DSS (n = 9), cGAL29 (n = 10), cGAL53 (n = 11). Data in (i, k–m) were analyzed by one-way ANOVA with multiple comparisons. Data are presented as mean ± SD. Statistically significant differences between groups are indicated with asterisks: *p < 0.05; ***p < 0.001; ****p < 0.0001.
In recent years, an increasing number of studies have indicated that the interaction between gut neurohormones and the immune system plays a pivotal role in the pathophysiology of IBD23,24. Galanin was initially isolated from the intestines of pigs and is widely distributed in the nerve fibers of the gastrointestinal tract25. It influences the release of neurohumoral substances, gut motility, smooth muscle contractility, fluid secretion, and intestinal ion flux26,27,28,29. Numerous experiments have attempted to explore the role of galanin and its receptors in IBD. For instance, the density of galanin-immunoreactive nerve fibers has been observed to be significantly elevated in dogs exhibiting moderate to severe IBD compared to their healthy counterparts30. Furthermore, in a rat model of acute colitis induced by trinitrobenzene sulfonic acid (TNBS), galanin has been shown to reduce the severity of colonic damage and inflammatory responses31,32. In a mouse model of colitis induced by dextran sulfate sodium (DSS), a higher level of GALR1 immunoreactivity was observed33. However, in DSS-induced colitis models using Galr1 knockout mice, colitis severity was similar to wild-type mice, although colonic fluid secretion was reduced34. GALR2 and GALR3 have been confirmed to be expressed in granulocytes within the colon tissue of CD and UC patients35. In DSS-induced colitis knockout mice, the absence of Galr3 significantly promoted weight loss, histologic damage, and inflammatory responses35. These findings highlight the potential significance of the GAL/GALR signaling pathways in treating IBD. However, the underlying mechanisms and signaling pathways still need to be fully understood.
By phylogenetic analysis of vertebrate galanin peptide sequences, we found a galanin splice variant from nonmammalian vertebrates that encodes a mature galanin peptide consisting of 53 amino acids (GAL53), with an additional 24 amino acids inserted after amino acid 13 of GAL29. Therefore, we analyzed the therapeutic effects of this new galanin peptide in chicken and mouse models of colitis induced by DSS and TNBS and compared it with the conserved GAL29. Additionally, we conducted in vitro cell experiments and in vivo animal studies to further investigate its receptors and signaling pathways.
Here, we show that chicken GAL53 is markedly downregulated in the inflamed colon and exhibits superior efficacy over the conserved GAL29 in alleviating colitis symptoms in both chicken and mouse models. We further identify GALR2 as the essential receptor for cGAL53 and demonstrate that its protective actions are mediated through a β-arrestin2-biased signaling pathway. This work not only reveals the physiological role of a novel galanin peptide but also provides new insights into the β-arrestin2-biased GALR2 agonizts for IBD treatment.
Results
Novel galanin peptide-GAL53 arising from alternative splicing in non-mammalian vertebrates
Galanin is a highly conserved peptide that emerged during the evolution from hemichordates to chordates and is present in all vertebrates (Fig.1a). In humans, the Galanin gene consists of six exons. Exon 2 encodes the signal peptide of ppGAL, exons 3 and 4 encode the mature peptide, and exons 5 and 6 encode the GMAP peptide (Fig. 1b). Nonmammalian vertebrates can produce an additional isoform of galanin through alternative mRNA splicing, resulting in an insertion of 24 additional amino acids between residues 13 and 14 of GAL29 (Fig. 1c). This isoform is encoded by an additional exon (3a) in the chicken Galanin gene, which is inserted between the original exons 3 and 4, and consists of seven exons in total (Fig. 1b). The galanin long splice variant is present in all examined vertebrate species, except mammals, cartilaginous fishes and cyclostomes, and the gene locus is conserved (Fig. 1a). Multiple sequence alignment indicates that the first 13 amino acids at the N-terminus of the GAL peptide are highly conserved across species (Fig. 1c). Furthermore, all vertebrate mature peptides are flanked by highly conserved dibasic proteolytic signals (KR) and, with the exception of primates, all mature peptides terminate with an amidation signal (Fig. 1c). In the subsequent analysis, we used real-time quantitative PCR (Q-PCR) to study the tissue expression and distribution characteristics of short and long galanin peptides in chickens. The Q-PCR results showed that cGal53 mRNA was highly expressed in the chicken intestine, followed by the pancreas and ovaries, with lower levels detected in the heart and lungs (Supplementary Fig. 1a).
The above results identified GAL53 as a variant in nonmammalian vertebrates that is expressed tissue specifically, implies potential functional significance.
Downregulation of cGal53 in a chicken intestinal inflammation model with enhanced therapeutic effects during colitis treatment of mice in comparison to cGAL29
Given the high expression of galanin in the chicken intestine (Supplementary Fig. 1a) and implications of the galanin system with colitis in rodents31,32, we established an intestinal inflammation model in chickens using a 4% DSS oral gavage. This DSS-induced intestinal inflammation model has been widely adopted in poultry research to investigate gut barrier dysfunction and inflammatory responses, with particular focus on the jejunum and ileum-critical segments for nutrient absorption in avian species36,37,38. Comparative analysis between the DSS-treated and control groups revealed a significant downregulation of cGal29 and cGal53 in the jejunum and an upregulation of cGalr2 in the ileum of chickens with intestinal inflammation (Fig. 1d, e and Supplementary Fig. 2b). In addition, we found that the expression of cGalr3 in the jejunum and ileum of the DSS-treated group decreased compared to the control group (Fig. 1e). There was no significant difference in the expression of cGalr1 and cGalr2b between the two groups (Supplementary Fig. 2a, c). To further investigate the role of galanin in chicken intestinal inflammation, we administered daily intraperitoneal injections of cGAL29 and cGAL53 into DSS-treated chickens throughout the induction period (Fig.1f). The results showed that both cGAL29 and cGAL53 ameliorated IBD-related symptoms in chickens, manifested as reductions of body weight loss, intestinal bleeding points and tissue damage (Fig. 1g–k and Supplementary Fig. 2d). Furthermore, galanin significantly attenuated the levels of various cytokines induced by DSS, including IL-6, TGF-β, IL-1β, and others (Fig. 1l, m and Supplementary Fig. 2e, f).
Due to the high conservation of galanin peptides, we extended our investigation to mammalian models to assess the therapeutic potential of cGAL53 in experimental intestinal inflammation. Two chemically induced colitis models mimicking aspects of human ulcerative colitis (UC) and Crohn’s disease (CD) pathologies were established to evaluate the effects of cGAL53. A DSS-induced colitis model was generated by administering 3% DSS in the drinking water, while the peptide treatment groups received daily intraperitoneal injections of hGAL30, cGAL29, or cGAL53, respectively (Fig. 2a). The DSS treatment led to a significant reduction of mouse body weight, colon length shortening and an increase of DAI score (Fig. 2b–e). However, treatment with galanin peptides significantly reversed these effects, with cGAL53 demonstrating superior therapeutic efficacy over the shorter galanin variants (cGAL29 and hGAL30) (Fig. 2b–e). DSS-induced high endoscopic and colon tissue damage scores were also mitigated in the galanin peptide-treated groups, with cGAL53-treated mice showing the most pronounced improvements (Fig. 2f–h). The expression of tight junction proteins, occludin and zona occludens-1 (ZO-1), was used to evaluate intestinal barrier integrity. Their protein and mRNA levels significantly decreased following DSS treatment but were markedly enhanced in colitis mice treated with cGAL53 (Fig. 2i and Supplementary Fig. 3a, b). Consistent with these findings, the FD4 permeability assay revealed that DSS treatment compromised intestinal barrier function, as evidenced by increased FD4 tracer levels in circulation. Notably, GAL peptide treatment effectively attenuated this DSS-induced hyperpermeability (Fig. 2j). For inducing colitis with 2,4,6-trinitrobenzene sulfonic acid (TNBS), which recapitulates transmural inflammation resembling human CD phenotypes, mice were administered TNBS intrarectally (Fig. 2m), which led to significant body weight loss, colon shortening, and increased histopathology scores compared to controls (Fig. 2n–r). Administration of cGAL53 proved to be more effective than cGAL29, nearly reversing the colon length and histopathological damage in C57BL/6 mice (Fig. 2n–r). In a dose-response study, cGAL29 at a concentration of 0.75 µM no longer improved weight loss and colon shortening in mice, while cGAL53 maintained its therapeutic effects on DSS-driven colitis, even at lower doses (Supplementary Fig. 4). To exclude potential side effects of GAL peptides under physiological conditions, we performed daily intraperitoneal injections of cGAL29 or cGAL53 in male mice for six consecutive days. Compared with the saline-injected control group, neither peptide affected body weight, water, and food intake, nor colon length, indicating good tolerability (Supplementary Fig. 5). Importantly, further analysis demonstrated that the superior therapeutic benefits of cGAL53 were maintained in female mice with DSS-induced colitis, indicating its gender-independent protective effects (Supplementary Fig. 6).
a Experimental scheme of DSS-induced colitis and peptide administration (3 μM) using 7–8-week-old mice. Silhouette from PhyloPic.org. b Change of body weight throughout the experimental period. Sample size for each group is as follows: control (n = 8), DSS (n = 12), cGAL29 (n = 11), cGAL29 (n = 6), cGAL53 (n = 8). c Disease activity index (DAI) (n = 6/group). d, e Representative images of the colon and the quantification of colon length (n = 6/group). Representative images of H&E staining (g) and HAI (f) analysis (n = 6/group). Scale bar = 50 μm. h Representative coloscopy images. i Expression of ZO-1 in the colon was assessed using IHC staining (n = 4/group). Scale bar = 50 μm. j Circulation FD4 levels (n = 4/group). Quantitative assessment of cytokine expression (Il6, Il1b, and Tnf) in the colons of DSS-induced mouse models (k) and serum levels measured by ELISA (l) (n = 5/group). m Experimental scheme of TNBS-induced colitis and peptide administration (3 μM) using 8–12-week-old mice. Silhouette from PhyloPic.org. n Body weight changes throughout the experimental period (n = 6/group). Representative images of the colon (o) and the quantification of colon length (p) (n = 6/group). q Representative images of H&E staining. Scale bar = 50 μm. r Representative coloscopy images. s Quantitative assessment of cytokine expression (Il6, Il1b, Tnf, Tgfb1, Ccl2 and Jun) in the colons of TNBS-induced mouse models (n = 5/group). Data are presented as mean ± SD. Statistical significance was determined by two-way ANOVA (for b, c, n) or by one-way ANOVA followed by a multiple comparisons test (for d–f, j–l, p, s). Statistically significant differences between groups are indicated with asterisks: *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001.
Collectively, these findings indicated that cGAL53 has therapeutic potential for intestinal inflammation in both chickens and mammals. Moreover, it has demonstrated superior therapeutic efficacy compared to the conserved GAL29 peptide, highlighting its potential as a more effective treatment option for IBD.
cGAL53 demonstrates significant anti-inflammatory effects during colitis treatment
Transcriptome sequencing was performed on the colon tissues of the aforementioned groups of DSS-induced colitis mice. DSS treatment induced changes in the expression of 8093 genes (Fig. 3a–c), and the upregulated genes were primarily enriched in pathways related to cytokine-cytokine receptor interaction, the PI3K-Akt signaling pathway, and focal adhesion, while downregulated genes were enriched in the cell cycle and biosynthesis of cofactors. These pathways were significantly modulated following treatment with cGAL29 and cGAL53 (Supplementary Fig. 7c). There are only 655 differentially expressed genes (DEGs) between the cGAL29 and cGAL53 group (Fig. 3a–c). Subsequently, KEGG and GO pathway enrichment analyses were conducted on these DEGs (Fig. 3d,e and Supplementary Fig. 7c). The cGAL53 group predominantly downregulated pathways associated with the IL-17 signaling pathway, TNF signaling pathway, inflammatory bowel disease, and chemokine signaling pathways, in contrast to the cCAL29 group (Fig. 3d, e). Furthermore, a comparative analysis of pathway changes in epithelial, adhesion, endothelial, and inflammation-related pathways across the control, DSS, cGAL29, and cGAL53 treatment groups highlighted the therapeutic effects of cGAL53 and cGAL29 on DSS-induced colitis, particularly in inflammation-related pathways (Fig. 3f–k and Supplementary Fig. 7a, b). Notably, cGAL53 demonstrated significantly greater reductions in inflammation-related pathways compared to cGAL29 (Fig. 3i). The expression levels of immune-related genes were significantly reduced in both cGAL29 and cGAL53 groups (Fig. 3k), and these expressions were validated in mouse colitis tissue by Q-PCR and ELISA (Fig. 2k, l and Supplementary Fig. 3c). Furthermore, the inhibitory effect of cGAL29 and cGAL53 on the expression of inflammatory cytokine genes were also evaluated using an in vitro Caco2 cell inflammation model (Supplementary Fig. 8a). As expected, both cGAL53 and cGAL29 reduced the expression of Il6, II23 and Tgfb1 induced by IL-1β and TNF-α treatment (Supplementary Fig. 8b). cGAL53 demonstrated a robust anti-inflammatory effect at low concentrations of 0.1 μM, while cGAL29 required a higher concentration of 10 μM to achieve similar anti-inflammatory effects (Supplementary Fig. 8b). These in vitro findings are consistent with our in vivo results, demonstrating that cGAL53 more effectively mitigates inflammatory responses than cGAL29. Taken together, these results indicated that cGAL53 exhibits superior protective effects against DSS-induced inflammation in both chickens and mice.
a, b Principal component analysis (PCA) of the RNA-seq data and the number of differentially expressed genes (DEGs) across all groups. c Venn diagrams displaying the number of commonly upregulated and downregulated genes across different groups. d, e KEGG and GO enrichment analyses were performed on the differentially expressed genes between the GAL53 and GAL29 groups. The figures depict pathways that ranked among the top 10 in at least one set of pairwise comparisons. f, g Gene set variation analysis (GSVA) analysis of signaling pathways related to epithelial function and adhesion. h, i Box-plot illustrating GSVA scores for pathways related to epithelial, adhesion, endothelial and immune (n = 2/group). Each point represents the GSVA score of a pathway. Data are presented as mean ± SEM, analyzed using two-sided Student’s t test. Box plots show median (center line) and 25th/75th percentiles, and whiskers extending to the most extreme data points within ±1.5× IQR. j, k Heatmaps of epithelial and inflammatory-related genes.
cGAL53 biased β-arrestin2 activation mediated by the galanin receptors
Galanin exerts its physiological effects by binding to three GPCRs, namely GALR1, GALR2, and GALR311. Phylogenetic analysis indicated that the galanin receptor clade is subdivided into the GALR1 and GALR2/3 clades, which is further differentiated into GALR1a and GALR1b within the GALR1 clade, and GALR3, GALR2a, and GALR2b within the GALR2/3 clade (Supplementary Fig. 9a). Notably, GALR1b is found exclusively in fish, GALR2b is present in nonmammalian vertebrates, and GALR3 is observed in most vertebrates (Supplementary Fig. 9b). Therefore, in chickens, there are four GALRs (cGALR1, cGALR2, cGALR2b, and cGALR3), whereas mammals have three (GALR1, GALR2, and GALR3). To better understand receptor specificity and signaling pathway bias between cGAL29 and cGAL53, we conducted pathway analysis in HEK293 cells and HTLA cells. Our luciferase results showed that both cGAL53 and cGAL29 can activate Gi (SRE-luc), Gs (CRE-luc), and Gq (NFAT-luc) signaling pathways of GALR2 and GALR3, but not GALR1 and cGAL2b (Fig. 4a–f and Supplementary Fig. 10). cGAL29 displayed a more robust activation of Gs and Gq pathways in GALR2 (Fig. 4a–c). These results were further confirmed by measuring intracellular calcium signal, and cAMP accumulation, and performing BRET assays in HEK293 cells following GAL stimulation (Fig. 4j–l and Supplementary Fig. 10h, i). We also assessed the β-arrestin2 (ARRB) pathway through Tango39 and BRET assays40. The results showed that cGAL53 significantly enhanced the ARRB2 recruitment of GALR1-R3 and GALR2b (Fig. 4g–i and Supplementary Fig. 10b, d, f). To investigate the critical amino acids responsible for the differential signaling pathways of cGAL53 and cGAL29, we truncated cGAL53 and found that the deletion of amino acids 38-53 abrogated the biased activation of the G protein pathways and β-arrestin2 recruitment downstream GALR2 (Fig. 4m, n). These results indicate that cGAL53 and cGAL29 exhibit differences in their receptor preference and signaling pathways, which may be crucial factors leading to their differing physiological functions.
HEK293 cells were transfected with 2 μg of GALR plasmids and 50 ng of a reporter gene plasmid. Peptides were added 36 h post-transfection, and luciferase activity was measured 12 h after stimulation. a–c G protein pathway activation of GALR2 after treatment with cGAL53 and cGAL29, where cGALR2, hGALR2, and mGALR2 represent chicken, human, and mouse GALR2. d–f G protein pathway activation of GALR3 in response to cGAL53 and cGAL29 treatment, as assessed by luciferase assay technical triplicate. g β-arrestin2 recruitment by GALR2 following cGAL53 and cGAL29 treatment, measured via the Tango assay. h β-arrestin2 recruitment by GALR3 after cGAL53 and cGAL29 treatment, using the Tango assay. i β-arrestin2 pathway activation of GALR2, measured via BRET assay. j Gq activation of GALR2 measured via BRET assay. Data are represented as mean ± SD. Calcium responses were measured by fluorescence intensity in GALR2 (k) and GALR3 (l) overexpressing cells following stimulation with various concentrations of GAL peptides. ATP (10 mM) and HBSS alone were used as positive and negative controls, respectively. m Schematic diagram of cGAL53 peptide truncations; note that in addition to the insert, alternative splicing leads to a different amino acid (Tyr versus His) just beyond the insertion. n Effects of cGAL53 truncations on the G protein pathway and β-arrestin2 recruitment to GALR2. Data in (a–j, n) are presented as mean ± SD from three independent experiments; data in (k, l) are presented as mean values from three independent experiments only.
GALR2 is the primary cGAL53 receptor in alleviating intestinal colitis
The dataset GSE235236 and GSE11223 were downloaded from the GEO database (https://www.ncbi.nlm.nih.gov/geo/) to identify potential therapeutic targets of galanin in IBD. The expression levels of Galr2 and Galr3 were analyzed utilizing ANOVA to determine statistical significance. Galr2 was highly expressed in the colonic tissues of UC and CD patients (Fig. 5a and Supplementary Fig. 11a). IHC staining further confirmed that GALR2 and GALR3 were primarily localized to mucosa surface epithelial cells in normal colon samples. In IBD patients, the GALR2-positive staining area was significantly increased, and immune cells also showed positive expression of GALR2 (Fig. 5b and Supplementary Fig. 11b). Consistently, increased GALR2 expression was observed in DSS-induced colitis mice (Fig. 5c and Supplementary Fig. 11c) and DSS-induced intestinal inflammation chickens (Fig. 1e), suggesting a potential role of GALR2 in the pathophysiology of inflammatory bowel conditions across species.
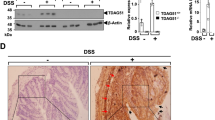
a GALR2 and GALR3 expression in GEO dataset GSE235236 (HC = 67, CD = 22, UC = 26). Box plots show median (center line) and 25th/75th percentiles, and whiskers extending to the most extreme data points within ±1.5× IQR. IHC staining of GALR2 and GALR3 in human UC colon (b, control: n = 6, UC: n = 3) and DSS-induced colitis mice (c). Arrows indicate epithelial (red) and immune cells (black). Scale bar = 50 μm. d Scheme of antagonist (10 nmol/mouse) and peptide (3 μM) administration in DSS-induced colitis (3% DSS, 8–12-week-old mice). Silhouette from PhyloPic.org. e Changes in body weight throughout the experimental period (n = 5/group). f Representative images of the colon and length quantification (n = 5/group). H&E staining (g) and HAI analysis (h) (n = 5/group, Scale bar = 100 μm). Quantitative evaluation of inflammation cytokine expression (Il1b, Il6, and Tnf) in colon after M871 (i) and SNAP37889 (j) treatment (n = 5/group). k DSS colitis and peptide administration (3 μM) scheme in Galr2-KO and Galr3-KO mice (7–8-week-old). Silhouette from PhyloPic.org. l Change of body weight throughout the experimental period. Sample size: WT-DSS and Galr3-KO groups (n = 8), WT-cGAL53 (n = 9), Galr2-KO groups (n = 7). m DAI analysis (n = 3). n Representative images of colon and colon length quantification (n = 6/group). o HAI analysis (n = 6/group). p Representative images of H&E staining (scale bar = 50 μm) and representative coloscopy images. q Expression of ZO-1 in the colon was assessed using IHC staining. Scale bar = 50 μm. r Circulation FD4 levels (n = 4/group). Quantitative assessment of cytokine expression (Il6 and Tnf) in the colons (s) and serum levels measured by ELISA (t) (n = 5/group). Statistical significance was determined using two-way ANOVA (for e, l, m), one-way ANOVA followed by a multiple comparisons test (for f, h–j), or a two-sided Student’s t test (for a, n, o, r–t). Data are presented as mean ± SD. Statistically significant differences between groups are indicated with asterisks: *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001.
To further elucidate the therapeutic target of cGAL53 for intestinal inflammation treatment, GALR2 and GALR3 inhibitors and respective knockout mice were used to determine which receptor is the functional target of cGAL53. We co-injected GALR2-selective antagonist M871 or GALR3-selective antagonist SNAP37889 with cGAL53 and observed that the addition of M871 inhibited the ability of cGAL53 to alleviate colitis41 (Fig. 5d). Compared to the cGAL53 group, the M871 + cGAL53 group exhibited accelerated weight loss, a shorter colon length, higher histological scores, and higher inflammatory cytokine mRNA levels (Fig. 5e–j). In contrast, in the SNAP37889 + cGAL53 group, cGAL53 was still effective in treating colitis symptoms in mice (Fig. 5e–h,j). Similarly, in an inflammation model using Caco-2 cells, the anti-inflammatory effects of cGAL53 were blocked by the GALR2-selective antagonist M871 but not by SNAP37889 (Supplementary Fig. 8c, d). Unlike cGAL53, both M871 and SNAP37889 exerted inhibitory effects on the therapeutic efficacy of cGAL29 (Supplementary Fig. 11d–f). Furthermore, the Galr2 and Galr3 knockout mice reaffirmed the aforementioned results. After exposure to 3% DSS for 8 days, the Galr2 knockout group and Galr3 knockout group exhibited severe symptoms (Fig. 5k–p and Supplementary Fig. 13a, b, e). Following treatment with cGAL53, both GALR3 knockout mice and WT mice showed a significant improvement in colitis symptoms, whereas Galr2 knockout mice did not exhibit any change (Fig. 5l–p). Compared with the Galr2 KO-DSS group, the expression of ZO-1 and occludin did not increase in the Galr2 KO-DSS+cGAL53 group (Fig. 5q and Supplementary Fig. 3d, e). Also, circulating FD4 tracer levels remained unchanged following cGAL53 treatment in Galr2-deficient mice (Fig. 5r). Furthermore, the expression of immune-related genes was not modulated in the absence of Galr2 (Fig. 5s, t). These results suggested that cGAL53 mediates its therapeutic effects on colitis in mice through the GALR2.
cGAL53 ameliorates intestinal colitis by enhancing the recruitment of ARRB2 but not G protein signaling downstream of GALR2 in epithelial cells
Considering that GALR2 serves as a therapeutic target for cGAL53 in managing IBD-related symptoms, and that cGAL53 predominantly stimulates the ARRB2 pathway associated with GALR2, we considered that cGAL53 modulates the GALR2/ARRB2 signaling pathway during IBD treatment. To validate this hypothesis, we generated Arrb2 flox mice and administered AAV8 viruses carrying a villin (Vil) promoter-controlled Cre (AAV8-Vil-cre) construct via enema to create gut epithelial cell-specific Arrb2 knockout mice (Arrb2ΔVil) (Fig. 6a and Supplementary Fig. 13c, f). These mice were then used to establish a DSS-induced colitis model to evaluate the impact of Arrb2 knockout on the therapeutic effects of GAL peptides (Fig. 6a). In Arrb2-cKO mice, the therapeutic effects of cGAL29 and cGAL53 in alleviating weight loss, colon shortening, intestinal damage, and inflammation, were significantly diminished compared to mice treated with the AAV control virus (Fig. 6b–g). Moreover, the mRNA expression of tight junction-related proteins, such as Tjp1 and Ocln, was not restored (Fig. 6h). These findings indicate that ARRB2 in epithelial cells is involved in mediating the anti-inflammatory and barrier-protective effects of galanin peptides in colitis. We also constructed GnaqΔVil mice to detect the impact of GALR2 on Gq signaling during galanin-mediated colitis treatment (Fig. 7a and Supplementary Fig. 13d, f). In these mice, the ability of cGAL29 and cGAL53 to alleviate weight loss, colon shortening, and intestinal damage, as well as inflammation was significantly reduced compared to mice treated with the AAV control-treated mice (Fig. 7b–g). However, the expression of Tjp1 and Ocln was unaffected, suggesting that Gq signaling in epithelial cells contributed specifically to the anti-inflammatory effects of GAL peptides in colitis (Fig. 7h). Together, these findings suggested that ARRB2 signaling downstream of GALR2 in endothelial cells plays distinct roles in mediating the therapeutic effects of GAL peptides in colitis treatment.
a AAV8-Vil-cre and AAV8-null were used to infect Arrbfl/fl mice (7–8 weeks) to generate intestinal epithelial-specific Arrb2 KO mice (ArrbΔVil) and control mice (Arrbfl/fl) for the 2% DSS-induced colitis model. Silhouette from PhyloPic.org. Change of body weight (b) and DAI (c) in Arrb2 cKO mice and control mice throughout the experimental period (n = 5/group). d Representative images of colon and quantification of colon length in Arrb2 cKO mice and control mice (n = 5/group). e Representative images of H&E staining. Scale bar = 200 μm. f ELISA was used to quantify serum IL-6 and IL-1β levels in each group of Arrb2 cKO mice (n = 5/group). g, h Quantitative evaluation of inflammation cytokines (Il6, Tnf, Il1b, and Tgfb1) and tight junction protein (Tjp1 and Ocln) expression in the colon of every group in Arrb2 cKO mice and control mice (n = 5/group). Statistical significance was determined using two-way ANOVA (for b, c) or one-way ANOVA followed by a multiple comparisons test (for d, e, f, h). Data are presented as mean ± SD. Statistically significant differences between groups are indicated with asterisks: *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001.
a AAV8-Vil-cre and AAV8-null were used to infect Gnaqfl/fl mice (7–8 weeks) to generate gut epithelial-specific Gnaq KO mice (GnaqΔVil) and control mice (Gnaqfl/fl) for the 2% DSS-induced colitis model. Silhouette from PhyloPic.org. Change of body weight (b) and DAI (c) in Gnaq cKO mice and control mice throughout the experimental period (n = 4/group). d Representative images of colon and quantification of colon length in Gnaq cKO mice and control mice (n = 5/group). e Representative images of H&E staining. Scale bar = 200 μm. f ELISA was used to quantify serum IL-6 and IL-1β levels in each group of Gnaq cKO mice (n = 5/group). g, h Quantitative evaluation of inflammation cytokine (Il6, Tnf, Il1b, and Tgfb1) and tight junction protein (Tjp1 and Ocln) expression in the colon of every group in Gnaq cKO mice and control mice (n = 5/group). Statistical significance was determined using two-way ANOVA (for b, c) or one-way ANOVA followed by a multiple comparisons test (for d, f, g, h). Data are presented as mean ± SD. Statistically significant differences between groups are indicated with asterisks: *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001.
Discussion
Darwinian medicine applies evolutionary principles to understand, e.g., the persistence of alleles that cause disease or finding new concepts for therapies42,43,44. For example, a peptide from the gila monster (Heloderma suspectum), extendin-4, with similarity to glucagon-like peptide 1 (GLP-1) had been shown to modulate glucose and lipid metabolism, and was developed to treat type 2 diabetes45. More recently, long-acting GLP-1 analogs liraglutide and semaglutide were developed, culminating in their novel application as obesity treatment46. In the present study, initially inspired by evolutionary medicine, we demonstrated that nonmammalian vertebrates express a longer form of galanin peptide (GAL53) in their intestines. Using synthetically produced peptides, we showed that GAL53 exhibits greater potency than the conserved shorter form (GAL29), as demonstrated by both in vitro and in vivo experiments. In addition, we elucidated the mechanism of action of GAL53 with its receptor GALR2, providing a theoretical framework for the application of avian GAL53 biased GALR2/β-arrestin2 pathway for the treatment of intestinal colitis.
Inflammatory bowel disease (IBD) includes ulcerative colitis (UC) and Crohn’s disease (CD); current IBD treatments focus on the suppression of the inflammatory response. In clinical practice, anti-inflammatory drugs, such as 5-aminosalicylic acid (5-ASA)47, corticosteroids48, and immunomodulators49 are widely used to reduce inflammation in IBD patients. Additionally, monoclonal antibody drugs targeting TNF-α, IL-12, and IL-23 are used in clinical settings50. In addition, kinase inhibitors, such as Janus kinase (JAK) inhibitors, are being applied to block specific signaling pathways to inhibit multiple pro-inflammatory cytokines, thereby reducing intestinal inflammation51. However, due to the lack of precision, high failure rates, and intolerances associated with the current therapeutic regimens, there is an urgent need to develop more effective and specific drugs to relieve the symptoms and improve quality of life in patients.
Galanin plays a crucial role in immune response, with its expression and associated receptors upregulated during acute inflammation in peripheral tissues8,52,53,54,55, highlighting its dynamic, context-dependent role within the immune system. For instance, exposure to copper sulfate increases galanin expression in zebrafish larvae, correlating with the reduction of lateral line damage and upregulation of inflammatory markers, such as Irg1, Il1b, and Tnf54. This suggests that galanin may play a role in immune response and inflammation. Research by Kofler et al. further implicates galanin in skin immunity, with increased expression observed in inflamed tissues8. Notably, siRNA-mediated knockdown of Galanin significantly suppresses the expression of IL-1β, IL-6, and cyclooxygenase (COX)−2, induced by UV irradiation56. Additionally, galanin is important in post-stroke inflammation, contributing to the infiltration of leukocytes and monocytes in the brain57. Current studies, particularly in the context of IBD, propose a protective, anti-inflammatory function for galanin. In a rat model of TNBS-induced acute colitis, galanin the severity of colonic damage and decreases inflammatory responses31,32. Furthermore, Galr3 knockout mice exhibit more severe colonic inflammation and aggravated histologic damage35. These findings suggest that galanin could potentially serve as a therapeutic agent for IBD. In our experiments, GAL29 not only alleviated intestinal inflammation symptoms in mouse colitis induced by DSS and TNBS but also reduced the expression of colonic inflammatory factors induced by DSS in chickens (Figs. 1 and 2), which is consistent with previous reports in the TNBS rat model31,32, indicating that the anti-inflammatory effect of GAL29 in treating intestinal inflammation is conserved. The mechanisms underlying the dysregulated expression of endogenous Galanin and its receptors during inflammation remain incompletely understood. Previous work by Marrero et al. demonstrated that Galr1 upregulation in DSS-induced colitis is associated with NF-κB activation, implicating this pathway in the inflammatory regulation of Galanin receptor expression33. In addition, Yang et al. identified a critical regulatory region (−320 to −300) in rat Galr2 promoter containing two putative ETS-1 binding sites that are essential for promoter activity58. These findings support the hypothesis that inflammation-responsive transcription factors, such as NF-κB and ETS-1, may contribute to the altered expression patterns of galanin receptors observed during colitis. Nevertheless, the upstream signaling pathways and transcriptional networks responsible for these changes remain to be fully elucidated and warrant further investigation. Our evolutionary analysis confirmed a longer galanin splice variant across various nonmammalian species, including teleost fish, amphibians, reptiles, and birds (Fig. 1). This variant is characterized by the insertion of 22–24 amino acids, which are encoded by an alternative exon between the third and fourth exons of the ancestral preprogalanin gene (Fig. 1). Although this new galanin splice variant has been reported in goldfish (Carassius auratus)59, European seabass (Dicentrarchus labrax)60 and Japanese quail (Coturnix japonica)61, its function had not been addressed. In this study, we amplified the long splice variant forms of galanin in chicken, revealing that the newly identified GAL53 isoform is predominantly expressed in the intestine (Supplementary Fig. 1). Notably, its expression is markedly downregulated in our broiler chicken DSS model (Fig. 1d). Importantly, GAL53 demonstrates superior protective effects against intestinal inflammation in birds and mammals (Figs. 1 and 2). Even at a concentration of 0.75 μM, it significantly enhances weight and colon length while reducing cytokine expression, highlighting its promising therapeutic potential in intestinal health and function (Supplementary Fig. 4).
We showed that cGAL53 and cGAL29 share the same receptor but differ in their ability to activate distinct signaling pathways (Fig. 4 and Supplementary Fig. 10). This discrepancy is the main reason for the therapeutic differences observed in intestinal inflammation treatment. Although the expression levels of GALR2 and GALR3 vary in humans, including healthy individuals and IBD patients, as well as in mice and chickens, a consistent trend was observed: GALR2 expression was significantly increased in colon samples from IBD patients, as well as in mice and chickens with intestinal inflammation (Fig. 5a–c; Fig. 1e and Supplementary Fig. 11a–c). Brunner’s experiments suggest that GALR3 is an ideal target for IBD treatment35. However, our study utilizing selective galanin receptor inhibitors and knockout mice demonstrated that GALR2 was the target receptor through which galanin exerts its therapeutic effects in IBD. Inhibition or deletion of GALR2 completely abolishes the effects of cGAL53 (Fig. 5). Our in vitro experiments showed that cGAL53 activated multiple G protein pathways (Gi, Gq, and Gs) and the ARRB pathway through GALR2 (Fig. 4 and Supplementary Fig. 10). Interestingly, cGAL29 activated the G protein pathways of both mouse and human GALR2 more strongly than cGAL53, while cGAL53 preferentially activated ARRB2 (Fig. 4 and Supplementary Fig. 10). AlphaFold3 was utilized for GALR2 structural prediction and GAL binding site analysis. Overall, both cGALR29, and cGAL53 exhibited a similar binding profile to mGALR2 (Supplementary Fig. 14). However, the ability of both cGALR29 and cGAL53 to recruit β-arrestin2 was also affected, when several predicted unique binding sites were mutated (Supplementary Fig. 14e). Despite this, we were unable to identify key sites responsible for the pathway differences between cGAL29 and cGAL53. The deletion of amino acids 38–53 in cGAL53 abolished the biased activation of G protein pathways and β-arrestin2 recruitment downstream of GALR2 (Fig. 4k, l). Although no direct interaction sites between the C-termini of GAL53 and GALR2 were predicted (Supplementary Fig. 14), we speculate that the truncation of amino acids 38–53 in the C-terminus may alter the conformation of the N-terminus, ultimately affecting the downstream activation of G protein pathways and β-arrestin2 recruitment through GALR2.
β-arrestins (ARRBs), comprising β-arrestin1 (ARRB1) and β-arrestin2 (ARRB2), are intracellular scaffolding proteins that serve as regulators and mediators of GPCR signaling. They facilitate the desensitization, internalization, and degradation of G protein-coupled receptors (GPCRs) and function as signaling molecules within the GPCR signal transduction process62,63. A growing body of evidence points to the functional involvement of β-arrestins in inflammation and autoimmune diseases64. However, their specific function in IBD remains a contentious issue. While a previous study found that deficiency of ARRB1 protects against experimental colitis65,66, Wu et al. have demonstrated that ARRB1 mediated mucosal protection by COX-1/PGE2/EP4 in colitis67. Recently, the same group reported that ARRB1 can safeguard intestinal tight junctions62. The function of ARRB2 in IBD is equally contentious. Studies by Ye et al. indicate that ARRB2 protein expression is significantly lower in active IBD than in remissive IBD and normal controls in the human species68. Conversely, Zeng et al. reported upregulation of ARRB2 in tissue samples from patients afflicted with UC or CD. Intriguingly, the absence of the ARRB2 gene in mice is associated with a significant reduction in intestinal inflammation, improving colitis symptoms, and decreasing mucosal apoptosis64. Our research has revealed that ARRB2 could be activated by galanin stimulation of GALR2 and that Arrb2-specific knockout in gut epithelial cells negated the therapeutic effects of both cGAL29 and cGAL53 on colitis (Fig. 6). Here, activation of ARRB2 appeared to play a protective role in IBD (Fig. 6). These findings provide a theoretical foundation for the treatment of colitis. Additionally, in epithelial cell-specific Gnaq knockout mice, the ability of galanin to promote the expression of ZO-1 and occludin was unaffected, indicating that the Gq pathway plays a role in the anti-inflammatory effects of galanin in IBD (Fig. 7). Our study demonstrated that GAL53 exerts protective effects in chemical models of IBD through bias the β-arrestin2 pathways downstream of GALR2 (Fig. 8). Furthermore, our study identified cGAL29 and cGAL53 as G protein- and β-arrestin2- biased agonizts of GALR2, respectively, which exhibited distinct therapeutic effects on intestinal inflammation. This underscores the significance of biased agonism in the treatment of intestinal colitis.
a, b The chicken Galanin gene undergoes alternative splicing to produce two variants, cGAL29 and cGAL53. The blue line represents cGAL29, while the red line indicates the amino acid insertions specific to cGAL53. cGAL29 exhibits stronger activation of G protein signaling pathways via GALR2. In contrast, cGAL53 significantly enhances the recruitment of ARRB2 to GALR2. This pathway bias enables cGAL53 to demonstrate greater therapeutic efficacy in treating intestinal inflammation in chickens and mice. This is achieved by suppressing the expression of inflammatory cytokines to reduce colonic inflammation and by enhancing the expression of ZO-1 and occludin to improve colonic barrier integrity, ultimately alleviating intestinal colitis.
In summary, our study has elucidated the mechanism of action of galanin with its receptor GALR2 for the potential treatment of IBD, providing a theoretical framework for the application of avian GAL53 biased GALR2/β-arrestin2 pathway for the treatment of IBD.
Methods
Reagents
Dextran sodium sulfate (DSS, MW:36000–50000) was purchased from Yeasen Biotechnology (Shanghai) Co., Ltd. 2,4,6-Trinitrobenzene sulfonic acid solution (TNBS) and fluorescein isothiocyanate-dextran (FD4, average mol wt 3000–5000) were purchased from Sigma. ATP-8 was obtained from Beyotime Biotechnology (Shanghai, China). Peptides were synthesized from Biotech Bioengineering (Shanghai, China) for functional assays, and the specific peptide sequences are shown in Supplementary Table 1. Peptide powders were dissolved in sterile water to prepare 1 mM stock aliquots. For luciferase and calcium assay, the stock solution was diluted to the desired concentrations using DMEM. For BRET and glosensor cAMP assay, dilutions were performed in HBSS buffer containing 20 mM HEPES and 5 µM coelenterazine-h. For in vivo studies, the stock was diluted to specified concentrations with sterile saline. GALR2 antagonist M871 (HY-P1130), GALR3 antagonist SNAP37889 (HT-2157), IL-1β (HY-P7028) and TNF-α (HY-P7058) were purchased from MCE. Chicken TNF-α (MM-0938O1), IL-1β (MM-36910O1) and IL-6 (MM-0521O1) ELISA kits, as well as mouse TNF-α (MU30030), IL-1β (MU30369) and IL-6 (MU30044) ELISA kit, were purchased from Jiangsu Meimian Industrial Co., Ltd. Fluo-4 DirectTM Calcium Assay kit was obtained from Invitrogen.
Patients and samples
Human paraffined-embedded colon sections from IBD patients and healthy individuals were obtained from the West China Hospital, Sichuan University. Detailed sample information is provided in Supplementary Table 2. The collection of human samples was approved by the local Ethical Committee and Review Board of West China Hospital (No. 2023[1081]). In accordance with the “Ethical Review Measures for Biomedical Research Involving Humans (2016)” of China, the Ethics Review Committee waived the requirement for informed consent for the use of human paraffined-embedded colon sections, which were leftover materials from diagnostic clinical biopsies that would have otherwise been discarded. This waiver was granted because the study involved minimal risk to participants, waiving consent would not adversely affect the rights and health of the subjects, and the research project did not involve personal privacy or commercial interests.
Animals
C57BL/6 mice, heterozygous Galr2 and Galr3 knockout mice, and Gnaqflox mice on a C57BL/6 background were purchased from GemPharmatech Co., Ltd (Nanjing, China). The Arrb2flox mice were constructed by Shanghai Model Organisms Center, Inc. (Shanghai, China). The homozygous and WT littermates used in this study were produced by breeding heterozygous parents. The mice were housed in plastic cages with five animals per cage and fed with standard chow and water ad libitum in specific pathogen free (SPF) grade animal rooms. Mice were kept under a 12 h-light/dark cycle-controlled atmosphere. Forty 21-day-old male Chinese yellow-feathered broilers were purchased from a local commercial hatchery, used for establishing the DSS-induced colitis model and 3-month-old females used for analyzing GAL expression patterns. The lighting schedule consisted of 24 h of light per day. All animal care and experimental procedures complied with the Animal Management Regulations of the Ministry of Health, China (Document No. 55, 2001) and were approved by the Science and Technology Department of Jiangsu Province [SYXK (SU) 2020-0047].
Plasmid construction
Human, mouse, and chicken galanin receptors were synthesized by General Bio Co., Ltd (Anhui, China) based on intron less NCBI sequences, and then cloned into pcDNA3.1-V5/His vector and Tango vector, respectively. The serum response element (SRE), cAMP response element (CRE), serum response factor response element (SRF-RE) and T-lymphocyte activation of nuclear factor-responsive element (NFAT-RE) luciferase reporter plasmids were obtained from Promega. The point mutation plasmids were constructed using the fast mutagenesis system obtained from TransGen Biotech (Beijing, China). The Glosensor reporter gene plasmid was purchased from Promega.
Cell culture, transfection and luciferase assay
HEK293 cells were purchased from Shanghai Fuxiang Biological Technology Co., Ltd (Shanghai, China). The HTLA cells, a HEK293T-derived cell line expressing a tetracycline-controlled trans- activator (tTA)-dependent luciferase reporter and a β-arrestin2, were a gift from Professor Zhenhua Shao. These cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM, Thermo-Fisher), supplemented with 10% fetal bovine serum (FBS, Gibco), 1% penicillin and 1% streptomycin (Gibco). For HTLA cells, puromycin (1 μg/mL, Gibco) and hygromycin (100 μg/mL, Gibco) were additionally supplemented. Caco2 cells were purchased from Shanghai Fuxiang Biological Technology Co., Ltd (Shanghai, China) and maintained in DMEM, supplemented with 20% FBS, 1% penicillin, 1% streptomycin and 1% non-essential amino acids (NEAA). All cells were cultured at 37 °C and in a 5% CO2 atmosphere.
For transient transection and luciferase assay, the HEK293 cells or HTLA cells were seeded in 48-well plates and transfected with empty vector-pcDNA3.1 or GALR (2 μg) using Lipofectamine-6000 (Beyotime, Shanghai, China). After 36 h, medium was replaced with serum-free medium containing galanin peptides and incubated for 12 h. Luciferase activities were determined 48 h after transfection using luciferase assay kits (Beyotime, Shanghai, China).
Glosensor cAMP Assay
HEK293 cells were seeded in 6-well plate at a density of 5 × 105 cells/well and incubate overnight. Cells were transfected with 0.5 µg GloSensor cAMP plasmid and 2 µg galanin receptor plasmid. After 48 h, the culture medium was aspirated and the cells were washed once with PBS, followed by trypsinization and centrifugation. Cell pellets were resuspended in 1× HBSS containing 3 µM D-luciferin potassium salt (light-protected). Aliquots of 90 µL cell suspension/well were transferred into a white opaque 96-well plate and incubated at 37 °C for 1 h to allow substrate uptake. After stimulation with 10 µL galanin (10−11–10−5 M) per well, incubation was allowed for 5–10 min, and luminescence measured.
Calcium assay
HEK293 cells were seeded in 96-well plates (black-walled, clear-bottom; LABSELECT, Cat# 11514) at a density of 1 × 10⁴–1 × 10⁵ cells per well. Cells were transiently transfected with 100 ng of galanin receptor plasmid per well using Lipofectamine-6000. Forty-eight hours post-transfection, intracellular calcium mobilization was measured in response to GAL peptide stimulation using the Fluo-4 Direct Calcium Assay Kit (Invitrogen) according to the manufacturer’s protocol. Briefly, cells were loaded with Fluo-4 dye for 1 h at 37 °C, followed by baseline fluorescence measurement (excitation 494 nm, emission 516 nm) using a PE EnVision 2105 multilabel plate reader. GAL peptide was then added at indicated concentrations (0.1, 1, and 10 μM) and real-time calcium flux was recorded. ATP (10 mM in HBSS) and HBSS alone were used as the positive and negative controls, respectively.
BRET assay
HEK293 cells were seeded at 5 × 105 cells per well in a 6-well plate and incubated overnight. The next day, cells were treated with PEI transfection reagent (YEASEN, 40816ES02) with the following DNA combinations per well: 0.5 μg Rluc8-Arrestin-3-Sp1, 1.0 μg mem-linker-citrine-SH3, and 2 μg galanin receptor plasmid. After one day of transfection, cells were seeded into white flat-bottom 96-well plates. The next day, the medium was removed and replaced with HBSS buffer, peptides were added to incubate at another 5 min in room temperature. The plate was read eight minutes from the start of coelenterazine-h injection using a BRET plate reader with detection filters set for Rluc8 (~485 nm) and Venus (~525 nm). For the Gq pathway assay, HEK293 cells were transfected with 1.0 μg receptor, 1.0 μg Gα-RLuc8, and 1.0 μg Gβ: Gγ-GFP2. Following peptide incubation, 20 μL of 25 μM coelenterazine 400a (Yeasen Biotechnology, China) was added, and plates were read immediately using emission filters set at 410 nm (Rluc8) and 515 nm (GFP2). Data analysis was performed using a simulation dose-response in GraphPad Prism 8.3.0.
RNA isolation and RT-qPCR
Total RNA was extracted from colon tissue using TRIzol reagent (Vazyme Biotech Co., Ltd) according to the manufacturer’s protocol, followed by quality control using capillary electrophoresis (NanoDrop 2000). RNA (1 μg) was reverse transcribed using a PrimeScript RT reagent kit with gDNA Eraser. Finally, target gene amplification was conducted using SYBR in the Bio-Rad Real-time system. Primers are listed in Supplementary Table 3. All primer were purchased from Sangon Biotech Co., Ltd (Shanghai, China). Fold changes in gene expression were normalized to mouse Gapdh and Actb or chicken Actb, respectively.
ELISA assay
Chicken jejunum and ileum tissue samples were homogenized in ice-cold PBS (pH 7.4) at a ratio of 1:9 (w/v). The homogenates were centrifuged at 5000 × g for 10 min at 4 °C, and supernatants were collected for cytokine analysis. The concentrations of pro-inflammatory cytokines (TNF-α, IL-1β, and IL-6) in tissue lysates were quantified using Jiangsu Meimian ELISA kits according to the manufacturer’s protocol.
DSS models
Male C57BL/6 mice or knockout mice (20 ± 2 g, 7–8 weeks of age) were randomly divided into different groups, the control group was given regular drinking water throughout the treatment periods, whereas the model groups were given 3% DSS in the drinking water ad libitum for 8 consecutive days. The intervention group were administered galanin (3 μM) daily via intraperitoneal injection. The control group received daily injections of an equal amount of physiological saline solution. For the female mouse model, 9–10-week-old C57BL/6 mice (20 ± 2 g) were randomly divided into four groups. The model groups were given 2.5% DSS in their drinking water ad libitum for 8 consecutive days. The intervention group received daily intraperitoneal injections of galanin (3 μM). The weight was recorded every day, and mice were eventually sacrificed. After the mice were euthanized, the colon was excised and washed using saline. The excised colon length was measured and photographed. Distal colon specimens were collected and fixed overnight in 4% formalin for morphological examination and immunostaining, while the remaining tissue was quickly frozen in liquid nitrogen and kept at −70 °C for subsequent experiments.
For the antagonist experiment, male C57BL/6 mice (20 ± 2 g, 7–8 weeks of age) were randomly divided into different groups. Both the control and peptide intervention groups received the same treatment protocols as previously described. GAL antagonists (M871 and SNAP 27889) were prepared according to the manufacturer’s instructions in a vehicle solution consisting of 10% DMSO, 40% PEG 300, 30% Tween-20, and 20% physiological saline. Antagonists were administered 30 min prior to peptide treatment.
Forty 21-day-old male broiler chickens were randomly divided into four groups: control group, DSS group, cGAL29 treatment group, and cGAL53 treatment group. The control group received a basal diet without DSS oral gavage, while the remaining three groups were fed a basal diet with DSS oral gavage from days 21 to 29. Each broiler chicken was orally administered 200 mL of 4% DSS per day, except for the control group. Additionally, the galanin treatment groups were i.p. injected daily with 100 μL of 50 μM peptide. During the entire experiment, the broilers had free access to food and water.
AAV delivery
Villin (Vil) promoter-controlled Cre (AAV8-Vil-cre) and control AAV(AAV8-Vil-null) constructs were ordered from Shanghai Genechem Co., Ltd (Shanghai, China). Prior to viral injection, 7–8-week-old Arrb2fl/fl and Gnaqfl/fl male mice were subjected to overnight fasting and subsequently anesthetized. A polyethylene catheter was then carefully inserted into the colon of the mice, extending 4 cm from the anus. Subsequently, the appropriate volume of virus (100 μL at a titer of 1E + 11 vg/mL) was administered via syringe in a slow and controlled manner. One-week post-AAV injection, mice were given 2% DSS in the drinking water ad libitum for 7 consecutive days to induce ulcerative colitis.
TNBS models
8–12-week-old male C57BL/6 mice were anesthetized using 1.5% isoflurane-mixed gas. The 100 μL of 2.5% TNBS (one volume of 5% (wt/vol) TNBS solution in H2O with one volume of absolute ethanol) solution was slowly injected into the lumen of the colon. The control group was treated only with 50% ethanol. Mouse weight was monitored daily, and the mice were sacrificed 72 h after colitis induction. Colon tissues were collected for histological analysis.
Evaluation of the disease activity index (DAI)
The DAI was calculated by combining scores for weight loss, stool properties, and hematochezia. The scoring criteria were as follows: weight loss (no change = 0; 1–5% = 1; 5–10% = 2; 10–20% = 3; >20% = 4), stool properties (normal = 0; soft stool = 1; moderate diarrhea = 2; diarrhea = 3), and hematochezia (no rectal bleeding = 0; slight rectal bleeding = 1; visible bloodstains on stool = 2; rectal bleeding = 3). The DAI = weight loss score + stool properties score + hematochezia score.
H&E staining and histopathology scores
Mouse colon tissues were fixed with 4% paraformaldehyde, embedded in paraffin, sectioned, and stained with H&E for histopathologic examination. Colon tissue sections were analyzed for epithelial damage, inflammatory infiltration, crypt loss and ulcerations. Histological evaluation of H&E-stained colonic sections was graded as follows: (a) severity of inflammation, none = 0; mild = 1; moderate = 2; severe = 3; (b) crypt damage, none = 0; basal 1/3 damaged = 1; basal 2/3 damaged = 2; only surface epithelium intact = 3; entire crypt and epithelium lost = 4; (c) sites of the lesion, none = 0; mucosal layer = 1; submucosal layer = 2; muscle layer = 3; transmural = 4; The histological scores were shown as the sum of the three evaluations with a maximal score of 11.2269.
Histological scoring of jejunum and ileum in chicken were the sum of 4 index scores, including the severity and extent of inflammatory cell infiltration, epithelial cell disruption, and villus height to crypt depth (VH:CD) ratio. The histological damage scoring criteria for chickens are as follows: (a) severity of inflammatory cell infiltration, none = 0; mild = 1; moderate = 2; severe = 3; (b) extent of inflammatory cell infiltration, none = 0; mucosa = 1; submucosa = 2; muscle layer = 3; (c) epithelial cell disruption, none = 0; mild = 1; moderate = 2; severe = 3; (d) VH:CD ratio, >6 = 0; 4–6 = 1; 2–4 = 2; <2 = 338.
Intestinal permeability method
To evaluate intestinal permeability, FITC-dextran (FD4, 25 mg/mL) was dissolved in 0.05 M PBS by vigorous vortexing. Mice were fasted for 12 h prior to and during the experiment. The FITC-dextran solution was administered via oral gavage at a dose of 20 mL/kg body weight. Three hours post-administration, blood samples were collected, and plasma was isolated. The plasma samples were diluted 1:2 with PBS, and 80 μL aliquots of the diluted standards and samples were transferred to a black 96-well plate. Fluorescence intensity was measured using a microplate reader (excitation: 490 nm, emission: 520 nm; bandwidth: 9 nm for both).
Immunohistochemistry
The sections of the intestine were deparaffinized, rehydrated, and subjected to heat-induced epitope retrieval for antigen unmasking. Endogenous peroxidase activity was blocked using hydrogen peroxide, and nonspecific binding was prevented by blocking with serum or protein blocking reagents. The sections were then incubated overnight at 4 °C with specific antibodies targeting ZO-1 (21773-1-AP, Proteintech, 1:1000), Occludin (13409-1-AP, Proteintech, 1:200), GALR2 (GTX100382, GeneTex, 1:200) and GALR3 (GTX108163, Genetex, 1:200). Subsequently, HRP-conjugated secondary antibodies were applied, and the positive signals were detected using a DAB kit (ZSGB-Bio, Beijing, China). Finally, the sections were counterstained with hematoxylin for visualization. Quantitative immunohistochemical analysis was performed using ImageJ software (NIH). The specificity of the GALR2 and GALR3 antibodies was validated in the colon of wild-type (WT) and Galr2-KO or Galr3-KO mice after DSS induction (Supplementary Fig. 12a). The specificity of the GALR2 antibody was further confirmed by immunofluorescence staining of brain sections from 8-week-old C57BL/6 mice (Supplementary Fig. 12b).
RNA-seq and analysis
Total RNA was isolated from colon tissue with Trizol reagent (Vazyme Biotech Co., Ltd). Sequence reads were obtained using BGIseq500 and successfully mapped to the mouse genome. For gene expression analysis, the matched reads were calculated and then normalized to fragments per kilobase million. Fold changes were calculated for all possible comparisons, and a 2-fold cutoff was used to select genes with expression changes. KEGG pathway analysis was performed using the R package, using significantly differentially expressed genes (P < 0.05) as target genes.
Alphafold3
The structure of mouse GALR2 was generated and predicted by AlphaFold3. Utilizing CavityPlus, potential binding pocket positions for each protein structure were identified. Subsequently, the chicken galanin peptides cGAL53 and cGAL29 were individually docked into the mGALR2 structural models, aligning with the critical amino acids that were identified within the predicted binding pockets.
Statistical analysis
In this study, bulk RNA-seq and publicly available GEO data were processed and analyzed using R. Results from these datasets are presented as mean ± SEM. All other quantitative data are expressed as mean ± SD. Statistical analyses were performed using GraphPad Prism (8.3.0) based on at least three independent experimental replicates. For comparisons between multiple groups, one-way or two-way ANOVA was applied as appropriate. Comparisons between two groups were performed using Student’s t test. Dose-response experiments were normalized and fitted using a nonlinear regression model (log[agonist] vs. response-variable slope, three parameters). A P-value of less than 0.05 was considered statistically significant.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
Publicly available datasets and databases used were following: colon biopsies from IBD patients and healthy controls (GSE11223, GSE235236). The RNA-sequencing generated in this study have been deposited in the NCBI database under accession code ID [PRJNA1334145]. All other data are available within the paper and its supplementary information. This manuscript does not report original code. Source data are provided with this paper.
References
Karwowski, C. A., Keljo, D. & Szigethy, E. Strategies to improve quality of life in adolescents with inflammatory bowel disease. Inflamm. Bowel Dis. 15, 1755–1764 (2009).
Sands, B. E. From symptom to diagnosis: clinical distinctions among various forms of intestinal inflammation. Gastroenterology 126, 1518–1532 (2004).
Guan, Q. A comprehensive review and update on the pathogenesis of inflammatory bowel disease. J. Immunol. Res. 2019, 7247238 (2019).
Monteleone, G., Fina, D., Caruso, R. & Pallone, F. New mediators of immunity and inflammation in inflammatory bowel disease. Curr. Opin. Gastroenterol. 22, 361–364 (2006).
Kaser, A., Zeissig, S. & Blumberg, R. S. Inflammatory bowel disease. Annu. Rev. Immunol. 28, 573–621 (2010).
Kaplan, G. G. The global burden of IBD: from 2015 to 2025. Nat. Rev. Gastroenterol. Hepatol. 12, 720–727 (2015).
Mitsukawa, K., Lu, X. & Bartfai, T. Galanin, galanin receptors, and drug targets. Exp.Suppl. 102, 7–23 (2010).
Kofler, B., Liu, M. L., Jacoby, A. S., Shine, J. & Iismaa, T. P. Molecular cloning and characterisation of the mouse preprogalanin gene. Gene 182, 71–75 (1996).
Vrontakis, M. E. Galanin: a biologically active peptide. Curr. Drug Targets CNS Neurol. Disord. 1, 531–541 (2002).
Parthiban, M. & Shanmughavel, P. Three dimensional modeling of N-terminal region of galanin and its interaction with the galanin receptor. Bioinformation 2, 119–125 (2007).
Webling, K. E., Runesson, J., Bartfai, T. & Langel, U. Galanin receptors and ligands. Front. Endocrinol. 3, 146 (2012).
Wang, B. et al. Differential activation of six galanin receptors by the spexin peptide in yellowtail kingfish (Seriola lalandi). Gen. Comp. Endocrinol. 359, 114629 (2024).
Wang, S., He, C., Hashemi, T. & Bayne, M. Cloning and expressional characterization of a novel galanin receptor. Identification of different pharmacophores within galanin for the three galanin receptor subtypes. J. Biol. Chem. 272, 31949–31952 (1997).
Kuteeva, E., Hokfelt, T., Wardi, T. & Ogren, S. O. Galanin, galanin receptor subtypes and depression-like behaviour. Cell Mol. Life Sci. 65, 1854–1863 (2008).
Depczynski, B. et al. Distribution and characterization of the cell types expressing GALR2 mRNA in brain and pituitary gland. Ann. N. Y. Acad. Sci. 863, 120–128 (1998).
Fathi, Z. et al. Cloning, pharmacological characterization and distribution of a novel galanin receptor. Brain Res. Mol. Brain Res. 51, 49–59 (1997).
Waters, S. M. & Krause, J. E. Distribution of galanin-1, −2 and −3 receptor messenger RNAs in central and peripheral rat tissues. Neuroscience 95, 265–271 (2000).
Smith, K. E. et al. Cloned human and rat galanin GALR3 receptors. Pharmacology and activation of G-protein inwardly rectifying K+ channels. J. Biol. Chem. 273, 23321–23326 (1998).
Lang, R. et al. Physiology, signaling, and pharmacology of galanin peptides and receptors: three decades of emerging diversity. Pharm. Rev. 67, 118–175 (2015).
Reyes-Alcaraz, A., Lee, Y. N., Yun, S., Hwang, J. I. & Seong, J. Y. Conformational signatures in beta-arrestin2 reveal natural biased agonism at a G-protein-coupled receptor. Commun. Biol. 1, 128 (2018).
Kim, D. K. et al. Coevolution of the spexin/galanin/kisspeptin family: spexin activates galanin receptor type II and III. Endocrinology 155, 1864–1873 (2014).
He, L. et al. Galanin ameliorates liver inflammation and fibrosis in mice by activating AMPK/ACC signaling and modifying macrophage inflammatory phenotype. Front. Immunol. 14, 1161676 (2023).
Gross, K. J. & Pothoulakis, C. Role of neuropeptides in inflammatory bowel disease. Inflamm. Bowel Dis. 13, 918–932 (2007).
El-Salhy, M. & Hausken, T. The role of the neuropeptide Y (NPY) family in the pathophysiology of inflammatory bowel disease (IBD). Neuropeptides 55, 137–144 (2016).
Tatemoto, K., Rokaeus, A., Jornvall, H., McDonald, T. J. & Mutt, V. Galanin-a novel biologically active peptide from porcine intestine. FEBS Lett. 164, 124–128 (1983).
Melander, T. et al. Distribution of galanin-like immunoreactivity in the gastro-intestinal tract of several mammalian species. Cell Tissue Res. 239, 253–270 (1985).
Ekblad, E., Rokaeus, A., Hakanson, R. & Sundler, F. Galanin nerve fibers in the rat gut: distribution, origin and projections. Neuroscience 16, 355–363 (1985).
Bauer, F. E. et al. Distribution and molecular heterogeneity of galanin in human, pig, guinea pig, and rat gastrointestinal tracts. Gastroenterology 91, 877–883 (1986).
Bauer, F. E. [Galanin: a new biologically active gastrointestinal neuropeptide]. Z. Gastroenterol. 28, 160–169 (1990).
Rychlik, et al. Galanin-immunoreactive nerve fibers in the mucosal layer of the canine gastrointestinal tract during inflammatory bowel disease. J. Vet. Res. 59, 143–148 (2015).
Talero, E., Sanchez-Fidalgo, S., Ramon Calvo, J. & Motilva, V. Galanin in the trinitrobenzene sulfonic acid rat model of experimental colitis. Int. Immunopharmacol. 6, 1404–1412 (2006).
Talero, E., Sanchez-Fidalgo, S., Calvo, J. R. & Motilva, V. Chronic administration of galanin attenuates the TNBS-induced colitis in rats. Regul. Pept. 141, 96–104 (2007).
Marrero, J. A., Matkowskyj, K. A., Yung, K., Hecht, G. & Benya, R. V. Dextran sulfate sodium-induced murine colitis activates NF-kappaB and increases galanin-1 receptor expression. Am. J. Physiol. Gastrointest. Liver Physiol. 278, G797–G804 (2000).
Matkowskyj, K. A. et al. Galanin contributes to the excess colonic fluid secretion observed in dextran sulfate sodium murine colitis. Inflamm. Bowel Dis. 10, 408–416 (2004).
Brunner, S. M. et al. Galanin receptor 3 attenuates inflammation and influences the gut microbiota in an experimental murine colitis model. Sci. Rep. 11, 564 (2021).
Dal Pont, G. C. et al. Novel models for chronic intestinal inflammation in chickens: intestinal inflammation pattern and biomarkers. Front. Immunol. 12, 676628 (2021).
Chen, Y., Zha, P., Xu, H. & Zhou, Y. An evaluation of the protective effects of chlorogenic acid on broiler chickens in a dextran sodium sulfate model: a preliminary investigation. Poult. Sci. 102, 102257 (2023).
Liu, L. et al. Establishment of an enteric inflammation model in broiler chickens by oral administration with dextran sulfate sodium. Animals 12, 3552 (2022).
Dogra, S., Sona, C., Kumar, A. & Yadav, P. N. Tango assay for ligand-induced GPCR-beta-arrestin2 interaction: application in drug discovery. Methods Cell Biol. 132, 233–254 (2016).
Kocan, M., Dalrymple, M. B., Seeber, R. M., Feldman, B. J. & Pfleger, K. D. Enhanced BRET technology for the monitoring of agonist-induced and agonist-independent interactions between GPCRs and beta-arrestins. Front. Endocrinol. 1, 12 (2010).
Barreto, S. G. et al. Galanin receptor 3-a potential target for acute pancreatitis therapy. Neurogastroenterol. Motil. 23, e141–e151 (2011).
Williams, G. C. & Nesse, R. M. The dawn of Darwinian medicine. Q. Rev. Biol. 66, 1–22 (1991).
Stearns, S. C. & Ebert, D. Evolution in health and disease: work in progress. Q. Rev. Biol. 76, 417–432 (2001).
Natterson-Horowitz, B. et al. The future of evolutionary medicine: sparking innovation in biomedicine and public health. Front. Sci. 1, 997136 (2023).
Deane, A. M., Chapman, M. J. & Horowitz, M. The therapeutic potential of a venomous lizard: the use of glucagon-like peptide-1 analogues in the critically ill. Crit. Care 14, 1004 (2010).
Holst, J. J. GLP-1 physiology in obesity and development of incretin-based drugs for chronic weight management. Nat. Metab. 6, 1866–1885 (2024).
Le Berre, C., Roda, G., Nedeljkovic Protic, M., Danese, S. & Peyrin-Biroulet, L. Modern use of 5-aminosalicylic acid compounds for ulcerative colitis. Expert Opin. Biol. Ther. 20, 363–378 (2020).
Bruscoli, S., Febo, M., Riccardi, C. & Migliorati, G. Glucocorticoid therapy in inflammatory bowel disease: mechanisms and clinical practice. Front. Immunol. 12, 691480 (2021).
Pithadia, A. B. & Jain, S. Treatment of inflammatory bowel disease (IBD). Pharm. Rep. 63, 629–642 (2011).
Deepak, P. & Loftus Jr, E. V. Ustekinumab in treatment of Crohn’s disease: design, development, and potential place in therapy. Drug Des. Dev. Ther. 10, 3685–3698 (2016).
Salas, A. et al. JAK-STAT pathway targeting for the treatment of inflammatory bowel disease. Nat. Rev. Gastroenterol. Hepatol. 17, 323–337 (2020).
Saban, M. R., Nguyen, N. B., Hammond, T. G. & Saban, R. Gene expression profiling of mouse bladder inflammatory responses to LPS, substance P, and antigen-stimulation. Am. J. Pathol. 160, 2095–2110 (2002).
Lang, R. & Kofler, B. The galanin peptide family in inflammation. Neuropeptides 45, 1–8 (2011).
Nowik, N., Przyborowska, A., Sienkiewicz, W. & Podlasz, P. Protective role of galanin during chemically induced inflammation in zebrafish larvae. Biology 10, 99 (2021).
Zhang, M. L., Fu, F. H. & Yu, L. C. Antinociception induced by galanin in anterior cingulate cortex in rats with acute inflammation. Neurosci. Lett. 638, 156–161 (2017).
Lee, M. J. et al. Galanin contributes to ultraviolet irradiation-induced inflammation in human skin. Exp. Dermatol. 26, 744–747 (2017).
Kriz, J. Inflammation in ischemic brain injury: timing is important. Crit. Rev. Neurobiol. 18, 145–157 (2006).
Yang, Y. et al. Characterization of the rat GAL2R promoter: positive role of ETS-1 in regulation of the rat GAL2R gene in PC12 cells. Mol. Neurobiol. 54, 4421–4431 (2017).
Unniappan, S., Lin, X. & Peter, R. E. Characterization of complementary deoxyribonucleic acids encoding preprogalanin and its alternative splice variants in the goldfish. Mol. Cell Endocrinol. 200, 177–187 (2003).
Martins, R. S. T. et al. Galanin isoforms by alternative splicing: structure, expression, and immunohistochemical location in the gonads of European sea bass. Gen. Comp. Endocrinol. 305, 113730 (2021).
Kohchi, C. & Tsutsui, K. Avian galanin: cloning of complementary DNAs and characterization of transcripts in different tissues. J. Exp. Zool. 287, 183–190 (2000).
Wu, S. et al. beta-arrestin1 protects intestinal tight junction through promoting mitofusin 2 transcription to drive parkin-dependent mitophagy in colitis. Gastroenterol. Rep.12, goae084 (2024).
Gao, H. et al. Identification of beta-arrestin2 as a G protein-coupled receptor-stimulated regulator of NF-kappaB pathways. Mol. Cell 14, 303–317 (2004).
Zeng, L. X. et al. Beta-arrestin2 encourages inflammation-induced epithelial apoptosis through ER stress/PUMA in colitis. Mucosal Immunol. 8, 683–695 (2015).
Lee, T. et al. Beta-arrestin-1 deficiency protects mice from experimental colitis. Am. J. Pathol. 182, 1114–1123 (2013).
Lee, T., Lee, E., Arrollo, D., Lucas, P. C. & Parameswaran, N. Non-hematopoietic beta-arrestin1 confers protection against experimental colitis. J. Cell Physiol. 231, 992–1000 (2016).
Peng, X. et al. COX-1/PGE(2)/EP4 alleviates mucosal injury by upregulating beta-arr1-mediated Akt signaling in colitis. Sci. Rep. 7, 1055 (2017).
Ye, X. et al. CCR5 expression in inflammatory bowel disease and its correlation with inflammatory cells and beta-arrestin2 expression. Scand. J. Gastroenterol. 52, 551–557 (2017).
Zhou, W. et al. Disulfiram with Cu(2+) alleviates dextran sulfate sodium-induced ulcerative colitis in mice. Theranostics 13, 2879–2895 (2023).
Acknowledgements
This work was supported by National Natural Science Foundation of China (32170498 to S.S.L, 32270438 to C.D); National Science and Technology Major Project for Prevention and Treatment of Cancers, Cardio-cerebrovascular Diseases, Respiratory Diseases, and Metabolic Diseases (2025ZD0551800 to C.D); National Science and Technology Major Project (2023ZD0506800 to C.D); National Key R&D Program of China (2021YFF0702000 to C.D); 1.3.5 Project for Disciplines of Excellence, West China Hospital, Sichuan University (ZYYC21011 to C.D); 1.3.5 Project of Center for High Altitude Medicine, West China Hospital, Sichuan University (GYYX24018 to C.D); National Clinical Research Center for Geriatrics, West China Hospital, Sichuan University (Z2023JC003 to C.D); Science and Technology Department of Sichuan Province (2022YFH0116 and TJZ2025EM08 to C.D); the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD to C.D); the “Kun lun Talents” of Qinghai-Top-Notch and Pioneering talent of Innovation and Entrepreneurship to C.D.
Author information
Authors and Affiliations
Contributions
C.D. and S.L. conceived and supervised the whole project; X.K., J.X., K.M., Y.C., X.Y., S.L., S.K., N.Y., T.Z., N.J., A.A., C.Z., N.U., and T.Z. conducted experiments; J.X., X.Y., and K.Z. performed the GEO data and RNA sequencing analysis; X.K., J.X., S.L., C.L., A.A., K.M., and C.D. performed data analysis and interpretation; S.L. and X.K. wrote the paper; J.B., S.L., and C.D. revised the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Lai, S., Kong, X., Xue, J. et al. A novel long-galanin peptide from non-mammalian vertebrates mitigates the inflammatory response in IBD models via the biased GALR2/β-arrestin2 pathway. Nat Commun 16, 11512 (2025). https://doi.org/10.1038/s41467-025-66335-1
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41467-025-66335-1