Abstract
Atrial fibrillation (AF) is more prevalent in patients with elevated interleukin (IL)-1β levels. Here we show that daily administration of IL-1β for 15 days sensitizes mice to AF, leading to fibrosis, accumulation of β-pleated sheet proteins in the left atrium, and systemic inflammation, resembling the pathophysiological changes observed in patients with AF. IL-1β administration creates a positive feedback loop, dependent on the IL-1 receptor (IL-1R) activity in cardiac resident macrophages. This results in increased caspase-1 maturation in the left atrium and elevated Il1b and Casp1 transcription in atrial macrophages. IL-1β treatment accelerated action potential and Ca2+ restitution in the left atrium, leading to action-potential shortening. This, along with increased caspase-1 maturation and IL-1R signaling, was essential for inducing AF. Lack of IL-1R in macrophages, but not cardiomyocytes, prevented IL-1β-induced AF sensitivity. By depleting recruited macrophages or deleting IL-1R specifically in cardiac resident macrophages, we further demonstrate that IL-1β/IL-1R signaling in these resident macrophages is responsible for increased AF susceptibility. These findings offer insights into the therapeutic potential of targeting IL-1β/IL-1R signaling in patients with AF and emphasize the importance of recognizing different underlying causes in this patient group.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
RNA-seq data are available in the Gene Expression Omnibus (GEO) under accession number GEO: GSE284265. Source data are provided with this paper.
Code availability
Custom code for FTIR spectroscopy analysis is available via Zenodo at https://zenodo.org/record/7338651#.Y3vqYHZByUl (ref. 51). Custom code for HRV analysis is available via Zenodo at https://zenodo.org/record/7335949#.Y3f7PHZByUk (ref. 51).
References
Chugh, S. S. & Gillum, R. F. Global burden of atrial fibrillation in developed and developing nations. Glob. Heart 9, 113–119 (2014).
Tsao, C. W. et al. Heart disease and stroke statistics—2022 update: a report from the American Heart Association. Circulation 145, e153–e639 (2022).
Heijman, J. et al. Atrial myocyte NLRP3/CaMKII nexus forms a substrate for postoperative atrial fibrillation. Circ. Res. https://doi.org/10.1161/CIRCRESAHA.120.316710 (2020).
Cabaro, S. et al. Epicardial adipose tissue-derived IL-1β triggers postoperative atrial fibrillation. Front. Cell Dev. Biol. 10, 893729 (2022).
Gomez, S. E. et al. Evaluation of the association between circulating IL-1β and other inflammatory cytokines and incident atrial fibrillation in a cohort of postmenopausal women. Am. Heart J. 258, 157–167 (2023).
Chen, X. et al. Downregulation of peroxisome proliferator-activated receptor-γ expression in hypertensive atrial fibrillation. Clin. Cardiol. 32, 337–345 (2009).
Wang, C. et al. Changes of interleukin-1beta and tumor necrosis factor-alpha of right atrial appendages in patients with rheumatic valvular disease complicated with chronic atrial fibrillation. Zhonghua Xin Xue Guan Bing Za Zhi 33, 522–525 (2005).
Hulsmans, M. et al. Recruited macrophages elicit atrial fibrillation. Science 381, 231–239 (2023).
Bohne, L. J. et al. Glucagon-like peptide-1 protects against atrial fibrillation and atrial remodeling in type 2 diabetic mice. JACC Basic Transl. Sci. 8, 922–936 (2023).
Yao, C. et al. Enhanced cardiomyocyte NLRP3 inflammasome signaling promotes atrial fibrillation. Circulation 138, 2227–2242 (2018).
Grune, J., Yamazoe, M. & Nahrendorf, M. Electroimmunology and cardiac arrhythmia. Nat. Rev. Cardiol. https://doi.org/10.1038/s41569-021-00520-9 (2021).
Nattel, S., Heijman, J., Zhou, L. & Dobrev, D. Molecular basis of atrial fibrillation pathophysiology and therapy: a translational perspective. Circ. Res. https://doi.org/10.1161/CIRCRESAHA.120.316363 (2020).
Zhou, J. et al. Comprehensive metabolomic and proteomic analyses reveal candidate biomarkers and related metabolic networks in atrial fibrillation. Metabolomics https://doi.org/10.1007/s11306-019-1557-7 (2019).
L¢pez-Canoa, J. N. et al. The role of fatty acid-binding protein 4 in the characterization of atrial fibrillation and the prediction of outcomes after catheter ablation. Int. J. Mol. Sci. https://doi.org/10.3390/ijms231911107 (2022).
Kugler, S. et al. Inflammasome activation in end-stage heart failure-associated atrial fibrillation. ESC Heart Fail. 9, 2747–2752 (2022).
Sikkel, M. B. et al. Hierarchical statistical techniques are necessary to draw reliable conclusions from analysis of isolated cardiomyocyte studies. Cardiovasc. Res. 113, 1743–1752 (2017).
Avula, U. M. R. et al. Heterogeneity of the action potential duration is required for sustained atrial fibrillation. JCI Insight https://doi.org/10.1172/jci.insight.128765 (2019).
Fakuade, F. E. et al. Altered atrial cytosolic calcium handling contributes to the development of postoperative atrial fibrillation. Cardiovasc. Res. 117, 1790–1801 (2021).
Reinhardt, F. et al. Abnormal calcium handling in atrial fibrillation is linked to changes in cyclic AMP dependent signaling. Cells https://doi.org/10.3390/cells10113042 (2021).
Kornyeyev, D. et al. Calsequestrin 2 deletion shortens the refractoriness of Ca2+ release and reduces rate-dependent Ca2+-alternans in intact mouse hearts. J. Mol. Cell. Cardiol. 52, 21–31 (2012).
Dobrev, D., Heijman, J., Hiram, R., Li, N. & Nattel, S. Inflammatory signalling in atrial cardiomyocytes: a novel unifying principle in atrial fibrillation pathophysiology. Nat. Rev. Cardiol. 20, 145–167 (2022).
Granowitz, E. V., Vannier, E., Poutsiaka, D. D. & Dinarello, C. A. Effect of interleukin-1 (IL-1) blockade on cytokine synthesis: II. IL-1 receptor antagonist inhibits lipopolysaccharide-induced cytokine synthesis by human monocytes. Blood 79, 2364–2369 (1992).
Dick, S. A. et al. Self-renewing resident cardiac macrophages limit adverse remodeling following myocardial infarction. Nat. Immunol. 20, 29–39 (2019).
Bain, C. C. et al. Constant replenishment from circulating monocytes maintains the macrophage pool in the intestine of adult mice. Nat. Immunol. 15, 929–937 (2014).
Yue, L. et al. Ionic remodeling underlying action potential changes in a canine model of atrial fibrillation. Circ. Res. 81, 512–525 (1997).
Brundel, B. et al. Induction of heat shock response protects the heart against atrial fibrillation. Circ. Res. 99, 1394–1402 (2006).
Ravelli, F. & Allessie, M. J. C. Effects of atrial dilatation on refractory period and vulnerability to atrial fibrillation in the isolated Langendorff-perfused rabbit heart. Circulation 96, 1686–1695 (1997).
Bosch, R. F. et al. Ionic mechanisms of electrical remodeling in human atrial fibrillation. Cardiovasc. Res. 44, 121–131 (1999).
Burashnikov, A. & Antzelevitch, C. Acceleration-induced action potential prolongation and early afterdepolarizations. J. Cardiovasc. Electrophysiol. 9, 934–948 (1998).
Suffee, N. et al. Impacts of a high-fat diet on the metabolic profile and the phenotype of atrial myocardium in mice. Cardiovasc. Res. 118, 3126–3139 (2022).
Atienza, F. & Jalife, J. Reentry and atrial fibrillation. Heart Rhythm 4, S13–S16 (2007).
Simats, A. et al. Innate immune memory after brain injury drives inflammatory cardiac dysfunction. Cell 187, 4637–4655.e26 (2024).
Alexanian, M. et al. Chromatin remodelling drives immune-cell fibroblast communication in heart failure. Nature 635, 434–443 (2024).
Amrute, J. M. et al. Targeting immune–fibroblast cell communication in heart failure. Nature 635, 423–433 (2024).
Sironi, M. et al. IL-1 stimulates IL-6 production in endothelial cells. J. Immunol. 142, 549–553 (1989).
Wu, Q. et al. Colchicine prevents atrial fibrillation promotion by inhibiting IL-1β-induced IL-6 release and atrial fibrosis in the rat sterile pericarditis model. Biomed. Pharmacother. 129, 110384 (2020).
Ajoolabady, A., Nattel, S., Lip, G. Y. H. & Ren, J. Inflammasome signaling in atrial fibrillation: JACC state-of-the-art review. J. Am. Coll. Cardiol. 79, 2349–2366 (2022).
Ridker, P. M. et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N. Engl. J. Med. https://doi.org/10.1056/NEJMOA1707914 (2017).
Ridker, P. M. et al. Effects of interleukin-1β inhibition with canakinumab on hemoglobin A1c, lipids, C-reactive protein, interleukin-6, and fibrinogen a phase IIb randomized, placebo-controlled trial. Circulation 126, 2739–2748 (2012).
Krisai, P. et al. Canakinumab after electrical cardioversion in patients with persistent atrial fibrillation: a pilot randomized trial. Circ. Arrhythm. Electrophysiol. 13, e008197 (2020).
Zhou, X. et al. Macrophage IL-1β mediates atrial fibrillation risk in diabetic mice. JCI Insight https://doi.org/10.1172/jci.insight.171102 (2024).
Song, J. et al. Chronic kidney disease promotes atrial fibrillation via inflammasome pathway activation. J. Clin. Invest. https://doi.org/10.1172/JCI167517 (2023).
Masotto, C. et al. Evidence for a different sensitivity to various central effects of interleukin-1 β in mice. Brain Res. Bull. 28, 161–165 (1992).
Newton, R. C., Uhl, J., Covington, M. & Back, O. The distribution and clearance of radiolabeled human interleukin-1 beta in mice. Lymphokine Res. 7, 207–216 (1988).
Rubsamen, R. et al. Anti-IL-6 versus anti-IL-6R blocking antibodies to treat acute ebola infection in BALB/c mice: potential implications for treating cytokine release syndrome. Front. Pharmacol. 11, 574703 (2020).
Monnerat, G. et al. Macrophage-dependent IL-1β production induces cardiac arrhythmias in diabetic mice. Nat. Commun. 7, 13344 (2016).
Kiss, N. et al. Quantitative analysis on ex vivo nonlinear microscopy images of basal cell carcinoma samples in comparison to healthy skin. Pathol. Oncol. Res. 25, 1015–1021 (2019).
Madeiro, J. P. V., Cortez, P. C., Marques, J. A. L., Seisdedos, C. R. V. & Sobrinho, C. R. M. R. An innovative approach of QRS segmentation based on first-derivative, Hilbert and wavelet transforms. Med. Eng. Phys. 34, 1236–1246 (2012).
Alarcon, M. M. L. et al. Cardiac arrhythmias after renal I/R depend on IL-1β. J. Mol. Cell. Cardiol. 131, 101–111 (2019).
Costa, M., Goldberger, A. L. & Peng, C.-K. Multiscale entropy analysis of biological signals. Phys. Rev. E 71, 021906 (2005).
Moreno-Loaiza, O. IL-1β sensitization to atrial fibrillation depends on caspase-1 expression. Zenodo https://doi.org/10.5281/zenodo.7338650 (2022).
Acknowledgements
This work was funded by Carlos Chagas Filho Foundation for Supporting Research in the State of Rio de Janeiro (FAPERJ) E26/200.396/2020, 200.067/2024 (to O.M.-L.) and Fundação Maria Emilia (to O.M.-L.), and E. Medei receives personal grant PQ CNPq and grants E-26/210.155/2020, E-26/203.169/2017, E-26/210.191/2020, E-26/210.253/2020, E-203913/2024, E-210040/2023, CNPq 310681/2018-9, 308097/2022-0, and 406761/2022-1 INTERAS – INCT. F.F. receives personal grant 2R01HL 143450-05A1. We thank A. Mattiazzi of Centro de Investigaciones Cardiovasculares, Universidad Nacional de La Plata, for the critical discussion regarding the manuscript revision. We thank the FACS facility and Luminex facility from the Rede de Plataformas Tecnológicas Fiocruz. V.C.S. receives personal grant Program of Immunology and Inflammation, Federal University of Rio de Janeiro. P.T.B. receives personal grant FAPERJ E26/203.983/2024, E26/210.617/2023, and CNPq 442211/2024-4.
Author information
Authors and Affiliations
Contributions
E. Medei, A.L.E., P.T.B., C.N.P., R.M.-B., and O.M.-L. designed the experiments. O.M.-L., T.P.S., E. Medei, O.F.S., M.V.T.P., N.A.d.O.J., L.R.d.S., R.P.C., C.M.d.F., R.M.-B., J.C.P.S., J.d.R.F., A.S.d.S., D.A., and R.R.L. recruited participants for the study and collected clinical information and blood samples. O.M.-L., N.V.-N., A.R.d.Y.G., M.d.A.S., E. Medei, and A.L.E. performed and analyzed electrophysiology experiments. O.M.-L., N.V.-N., A.B.C.-J., T.P.S., E. Monteiro, T.H.K.-B., D.B.M., T.G.-S., T.M.C., F.M.d.S., and G.V.L.d.S. performed and analyzed FACS. V.C.S., S.S.G.D., L.M., H.M.-F., D.F.O., and B.C.B. performed western blots. O.M.-L., L.C., and M.J.C.-C. performed and analyzed mice multiplex assay. V.C.S., S.S.G.D., and P.T.B. performed and analyzed human multiplex assay. V.C.S., S.S.G.D., and P.T.B. performed cell culture and quantitative PCR from BMDM. O.M.-L. and J.P.d.V.M. analyzed RR series and HRV. M.d.A.S., T.P.S., A.B.P., L.P., E. Monteiro, M.C.A., and S.B.S. genotyped and performed experiments in Cre Lox mice. S.R.B. analyzed echocardiography exams. O.M.-L. and R.C.P. analyzed arrhythmias in mice after burst pacing. M.C.C., M.S.C.-R., and H.d.S.M. performed FTIR and SHG microscopy. O.M.-L., I.U., and F.F. recorded and analyzed optical mapping. O.M.-L., C.N.P., and E. Medei redacted the manuscript. All authors critically reviewed the manuscript and approved its final version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Cardiovascular Research thanks Dawood Darbar, Francis L. Martin, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
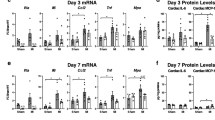
Extended Data Fig. 1 Comparative mRNA expression in atrial samples of mice injected with saline or IL1β for 15 consecutives days.
a. Fold change was calculated dividing mean TPM values of IL1β -treated by the control group. Genes from the list of interest (n = 72) with absolute log2-fold change higher than 0.5 were displayed in red. The cumulative expression level of the genes is depicted in the x-axis as the sum of all the transcript per million of the four samples. b. Gene-set enrichment analysis interrogating KEGG data base. c. Gene-set enrichment analysis interrogating Reactome data base. d. Gene-set enrichment analysis interrogating Wikipathways data base. The top 10 up-regulated pathways are shown in blue and the top 10 down-regulated in orange (b-d).
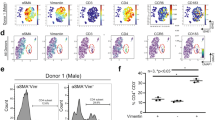
Extended Data Fig. 2 IL-1β injection increases circulating neutrophils and macrophages.
a, Mice circulating biomarkers. b, Scheme of experimental design for leukocyte subsets in LA at 15 days post-injections. c, Mouse atria immune cells profile. All the central lines in the box plots represent the median, the box limits represent the first and third quartiles and the whiskers denote the minimum and maximum values. P values were calculated with Mann-Whitney’s test (two-tailed) (a), and unpaired Student’s t-test (two-tailed) (c).
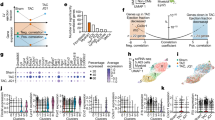
Extended Data Fig. 3 Human circulating biomarkers.
Patients in sinus rhythm (SR) or in atrial fibrillation (AF) circulating biomarkers. All the central lines in the box plots represent the median, the box limits represent the first and third quartiles and the whiskers denote the minimum and maximum values. P values were calculated with Mann-Whitney’s test (two-tailed).
Extended Data Fig. 4 IL-1β injection does not produces maturation of IL-1β in left atria of Casp1-/-.
a, Serum levels of IL-6 in saline- and IL-1β–injected Casp1–/– mice. b, Western blot of IL-1β/pro-IL-1β in Casp1-/- mice. Quantitation levels were normalized to glyceraldehyde 3-phosphate dehydrogenase (GAPDH) loading control. Each sample represents one mouse heart; 5 saline-injected and 6 IL-1β-injected, samples were analyzed. All the central lines in the box plots represent the median, the box limits represent the first and third quartiles and the whiskers denote the minimum and maximum values. P values were calculated with Mann-Whitney’s test (two-tailed) (a) and Student’s t-test (two-tailed) (b).
Extended Data Fig. 5 PKA and CaMKII expressions and the effect of Nifedipine on LA.
a, Western blot of Calcium-Calmodulin Kinase II δ (CaMKII), phospho-CaMKII Thr287 (p-CaMKII) and oxidized-CaMKII Met 281/282 (oxi-CAMKII). Quantitation levels were normalized to vinculin, used as loading control. Each sample represents one mouse heart; 5 saline-injected and 3 IL-1β-injected samples were analyzed. b Western blot of catalytic subunit of Protein kinase A (PKA). Quantitation levels were normalized to glyceraldehyde 3-phosphate dehydrogenase (GAPDH) loading control. Each sample represents one mouse heart; 8 saline-injected and 8 IL-1β-injected samples were analyzed (see the second membrane in uncropped Material). c, APDs before and after Nifedipine perfusion (at 30%, 50%, 70%, and 90% of repolarization). Data are presented as individual values before and after condition. Data in a and b are presented as the central lines in the box plots represent the median, the box limits represent the first and third quartiles and the whiskers denote the minimum and maximum values. Data in c is presented as individual values before and after condition. P values were calculated with unpaired Student’s t-test (two-tailed) (a,b), and paired samples Student’s t-test (two-tailed) (c).
Extended Data Fig. 6 Action Potential after 4-aminopyridine (4-AP) and atropine (Atrop) perfusion.
a, Graphical scheme of action potential recording after 15 min of 4-AP 2.5 mM perfusion. b, Representative traces of AP from saline and IL-1β groups perfused with Tyrode solution or 4-AP. c, Action potential duration (APD) at 30%, 50%, 70% and 90% of repolarization, from saline and IL-1β groups during perfusion with Tyrode (Tyr) or 4-AP. Data are presented as individual values before and after condition. d, Graphical scheme of action potential recording after 15 min of Atropine 10 µM perfusion. e, Representative traces of AP from saline and IL-1β groups perfused with Tyrode solution or atropine. f, APD at 30%, 50%, 70% and 90% of repolarization, from saline and IL-1β groups during perfusion with Tyrode (Tyr) or atropine (Atrop). Data are presented as individual values before and after condition. P values in c and f were calculated with paired samples Student’s t-test (two-tailed).
Extended Data Fig. 7 IL-1β injection increases arrhythmia susceptibility in a mechanism dependent of Casp1 expression and not IL-6.
a, graphical scheme of experiments. Casp1-/- mice were treated for 15 days with saline or IL-1β subcutaneously (SC). b, representative EKG traces from saline and IL-1β treated mice. c, EKG parameter analyzed: RR interval, p wave duration, and PR interval. d, representative action potential traces from saline and IL-1β treated Casp1-/- mice. e, action potential duration (APD) at 30%, 50%, 70% and 90% of repolarization, from saline and IL-1β treated mice. Lower inset panel show adjusted mean ± 95% C.I.f, triggered activities in Casp1-/- mice. g, graphical scheme of IL-6 restitution in Casp1-/- mice injected with IL-1β. h, representative EKG trace in sinus rhythm after burst-pacing in Casp1-/- mice injected with IL-1β and IL-6. i, AF inducibility after burst-pacing in Casp1-/- mice injected with IL-1β and IL-6. All the central lines in the box plots represent the median, the box limits represent the first and third quartiles and the whiskers denote the minimum and maximum values. Categorical data are presented as bar plots (f,i). P values were calculated with Student’s t-test (two-tailed) (c), hierarchical analysis with mixed effect model (e), and Fisher’s exact test (f,i) (two-tailed).
Extended Data Fig. 8 IL-1β injection increases arrhythmia susceptibility through activation of IL-1R.
a, graphical scheme of experiments. IL-1r-/- mice were treated for 15 days with saline or IL-1β subcutaneously (SC). b, representative EKG traces from saline and IL-1β treated mice. c, EKG parameters analyzed: RR interval, p wave duration, and PR interval. d, representative action potential traces from saline and IL-1β treated mice. e, action potential duration (APD) at 30%, 50%, 70% and 90% of repolarization, from saline and IL-1β treated mice. Lower inset panel show adjusted mean ± 95% C.I.All the central lines in the box plots represent the median, the box limits represent the first and third quartiles and the whiskers denote the minimum and maximum values. Categorical data are presented as bar plots (f). P values were calculated with unpaired Student’s t-test (two-tailed) (c), hierarchical analysis with mixed effect model (e), and Fisher’s exact test (two-tailed) (f).
Extended Data Fig. 9 IL-1β injection does not produces maturation of IL-1β in left atria of Csf1rCre IL-1rfl/fl.
a, graphical scheme of experiments. Csf1rCre IL-1rfl/fl mice were treated for 15 days with saline or IL-1β subcutaneously (SC). b, Western blot of IL-1β/pro-IL-1β in Csf1rCre IL-1rfl/fl mice. Quantitation levels were normalized to glyceraldehyde 3-phosphate dehydrogenase (GAPDH) loading control. Each sample represents one mouse heart; 5 saline-injected and 6 IL-1β-injected samples were analyzed. All the central lines in the box plots represent the median, the box limits represent the first and third quartiles and the whiskers denote the minimum and maximum values. P values were calculated with Student’s t-test.
Supplementary information
Supplementary Information
Supplementary Tables 1–7, Figs. 1–9, and unprocessed western blots and gels.
Supplementary Data 1
Supplementary data for Fig. 7.
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 5
Statistical source data.
Source Data Fig. 6
Statistical source data.
Source Data Fig. 7
Statistical source data.
Source Data Fig. 8
Statistical source data.
Source Data Extended Data Fig. 2
Statistical source data.
Source Data Extended Data Fig. 3
Statistical source data.
Source Data Extended Data Fig. 4
Statistical source data.
Source Data Extended Data Fig. 5
Statistical source data.
Source Data Extended Data Fig. 6
Statistical source data.
Source Data Extended Data Fig. 7
Statistical source data.
Source Data Extended Data Fig. 8
Statistical source data.
Source Data Extended Data Fig. 9
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Moreno-Loaiza, O., Soares, V.C., de Assumpção Souza, M. et al. IL-1β enhances susceptibility to atrial fibrillation in mice by acting through resident macrophages and promoting caspase-1 expression. Nat Cardiovasc Res 4, 312–329 (2025). https://doi.org/10.1038/s44161-025-00610-8
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s44161-025-00610-8