Abstract
Endometrial receptivity is a critical determinant of embryo implantation and early pregnancy success; however, current methods for assessing endometrial receptivity remain poorly validated and insufficiently reliable for clinical application. Here, we establish a patient-derived vascularised endometrium-on-a-chip (EoC), successfully replicating the dynamic microenvironment and both temporal and spatial architecture of native endometrial tissue. Using our EoC, we develop a clinically relevant endometrial receptivity scoring system, ERS2, which integrates molecular profiling of established receptivity markers with quantitative analyses of angiogenesis. The ERS2 enables personalised assessment of endometrial health and implantation potential, addressing inter-patient variability often overlooked by conventional techniques. By leveraging our EoC to therapeutic monitoring, we observe progressive restoration of the endometrial microenvironment following platelet-rich-plasma treatments, highlighting the translational utility of our model. This study represents the innovative application of a patient-derived EoC and scoring system to assess receptivity, offering personalised infertility management and advancing targeted therapies in reproductive medicine.
Similar content being viewed by others
Introduction
The endometrium is an intricate and dynamic organ that undergoes cyclic remodelling in synchrony with the female reproductive hormones. This remodelling includes proliferative, secretory, and menstrual shedding (in the absence of pregnancy) phases1. The endometrial layer plays a pivotal role in embryo implantation, a process that depends on precise positioning within the endometrium and synchronisation with the appropriate phase of the menstrual cycle2. Endometrial receptivity refers to the state during which the endometrium supports blastocyst adhesion to the epithelium, penetration into the stroma, and interaction with the maternal vasculature3. In humans, the ‘window of implantation (WOI)’, typically occurring between the 20th to 24th days of a standard 28-day menstrual cycle, is recognised as the optimal timeframe for embryo attachment. Notably, this period is characterised by a tightly coordinated interplay of various molecular pathways involving adhesion molecules, cytokines, and growth factors that cooperatively establish an appropriate environment favourable for the embryo implantation4. A series of molecular, cellular, and structural changes in the endometrium of the WOI are regulated by a combination of hormonal signals, particularly progesterone, and factors, such as vascular endothelial growth factor (VEGF), which promotes angiogenesis, further enhancing the endometrial receptivity to support successful implantation5. Hence, endometrial angiogenesis is among the main factors affecting endometrial receptivity and plays a critical role in the endometrial response to embryo implantation6. In addition, studies on factors contributing to endometrial receptivity highlights integrins as key biomarkers, particularly integrin αvβ3, whose expression is modulated during the luteal phase of the menstrual cycle. The interaction between integrin αvβ3 and its ligand osteopontin (OPN) is focused on, as it is believed to be pivotal in mediating the adhesion of the embryo to the endometrial epithelium7.
A critical step in assisted reproductive technology (ART) is the successful transfer of a high-quality embryo into the receptive endometrium8. For implantation and subsequent pregnancy to occur, it is essential to ensure both an optimal embryo and a well-prepared endometrial environment, as well as precise synchronisation between the two9. Various approaches have been proposed to evaluate endometrial receptivity, including the assessment of endometrial thickness10, the evaluation of endometrial pattern11, the use of endometrial receptivity array (ERA)12, measurement of endometrial blood flow13, and uterine artery Doppler assessments14. While these techniques have contributed to our understanding of endometrial receptivity, their ability to provide a comprehensive and accurate assessment at the time of the endometrium of WOI remains limited. Current clinical tools for evaluating endometrial receptivity are still largely based on the subjective judgement of clinicians, rather than on robust and evidence-based guidelines. Therefore, accurate identification of the receptive endometrium remains a significant challenge in ART, highlighting the need for further advancements in both diagnostic techniques and clinical practice.
Endometrial thickness is a commonly assessed parameter in in vitro fertilisation (IVF) cycles, with a measurement of 9-14 mm in thickness considered optimal for successful embryo transfer and live birth15. However, assessing endometrial receptivity based solely on thickness has certain limitations, and it remains unclear whether pregnancy and live birth rates plateau at a specific thickness or continue to increase as the thickness increases. Although endometrial perfusion and uterine artery Doppler assessments have been explored as potential indicators of endometrial blood flow, their impact on pregnancy outcomes in IVF cycles remains unclear16. Recently, the ERA has emerged as a promising tool to improve the accurate assessment of endometrial receptivity17. ERA is a test for measuring the gene expression profile of the endometrial lining to assess whether the endometrium is sufficiently ripe for embryo implantation. By identifying the optimal timing for progesterone administration prior to embryo transfer, ERA has the potential to improve the chances of successful implantation and pregnancy17. In addition to the ERA, imaging techniques, such as transvaginal ultrasound, offer valuable insights into the structural and vascular features of the endometrium18. However, there are many limitations to reflecting the patient’s endometrial environment, particularly in fully capturing the dynamic and real-time changes and the phenotypical interactions between the blood vessels and tissues within the endometrial environment19.
Following the introduction of microfluidics, in which complex biological systems are simulated and intensively investigated in a controlled setting, three-dimensional (3D) microfluidic platforms, known as organ-on-a-chip models by virtue of their capacity to recapitulate physiological processes within a 3D matrix, have emerged as effective tools to simulate organ-specific microenvironments20. A significant advantage of this method is that it monitors cell-to-cell interactions in real-time under variable conditions of fluid shear stresses and flow, which enables the differentiation and extended culturing of various cells20,21. Owing to these advantages, 3D microfluidic platforms have been applied to improve the accurate replication of in vivo tissue-specific cellular microenvironments, enabling the simulation of pathophysiological conditions and clinical mimicry in various organs including the lungs22,23,24, gut25, placenta26, liver27,28, heart29,30, and endometrium31,32,33. Building on these advancements, our group recently developed a microengineered vascularised endometrium-on-a-chip (EoC) model that incorporates the epithelial, stromal, and blood vessel layers to closely mimic the in vivo endometrial microenvironment31. The EoC successfully recapitulated the crucial features of endometrial vasculo-angiogenesis and hormonal responses, reflecting the proliferative and secretory phases of the menstrual cycle. This innovative model holds significant promise for drug screening and for studying the pathophysiology of the endometrium under dynamic and physiologically relevant conditions31.
In this study, we develop an innovative, patient-derived vascularised EoC model that incorporates three essential structural layers: the epithelium composed by endometrial epithelial organoids, stroma, and blood vessels. This model is rigorously validated across species, using both mouse and human endometrial samples, to ensure a faithful representation of the endometrial microenvironment, effectively capturing both the temporal and spatial dynamics critical to endometrial function in vivo. By analysing specific molecular expression patterns, particularly focusing on the essential endometrial receptivity markers, integrin αvβ3 and its binding ligand OPN, and conducting detailed angiogenic phenotypic assessments, such as angiogenic sprouting and vessel formation within the patient-derived vascularised EoC models, we successfully develop an endometrial receptivity scoring system (ERS2). We subsequently apply the ERS2 to assess endometrial receptivity in patients admitted to an infertility clinic, offering a perspective for personalised endometrial receptivity assessment. Furthermore, we expand our study to screen various drug candidates aimed at enhancing endometrial function, with a particular focus on patients with uterine synechiae. Notably, progressive improvements in the patient’s endometrial microenvironment are observed following three consecutive treatments with platelet-rich plasma (PRP), underscoring the clinical relevance of this platform. Overall, our microengineered EoC model, combined with the ERS2, represents the personalised tool capable of evaluating the endometrial microenvironments in patients to predict successful pregnancy outcomes, positioning it as a potentially transformative advancement in personalised translational infertility treatment.
Results
Design of the patient-derived vascularised endometrium-on-a-chip
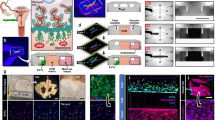
The patient-derived vascularised EoC, established as previously described31, was designed to recapitulate individual endometrial environments allowing physiological networks among the multi-layered endometrial tissues and maintaining their temporal phenotypes (Fig. 1). The functionalis of human endometrium, comprising epithelial, stromal, and endothelial layers, undergoes cyclic changes during the menstrual cycle, including the proliferative and secretory phases (Fig. 1a–i and ii). It is particularly crucial at the initial maternal-foetal interface when embryo implantation is initiated (Fig. 1a–iii). The EoC consists of three compartments, namely the epithelium, stroma, and endothelium structured by two parallel microchannels, endometrial fibroblast, stromal, and endothelial channels, to mimic the multi-layered and vascular features of the endometrium (Fig. 1b). The EoC construction was initiated by plating stomal cells mixed with fibrin gel into each of the endometrial stromal and fibroblast channels, allowing gelation to create the stromal compartment of the endometrium (Fig. 1c-Step ➀). Subsequently, human umbilical vessel endothelial cells (HUVECs) within a mixture of fibrin gel were placed into the endothelial cell channel adjacent to the endometrial stromal channel, forming a fully endothelialised channel that could generate the vasculature of the endometrium (Fig. 1c- Step ➁). After 24 h, the endometrial epithelial layer was formed by placing the endometrial epithelial organoids on top of the endometrial stromal channel (Fig. 1c- Step ➂). Importantly, freshly dissociated endometrial epithelial organoids and stromal cells obtained from biopsied tissues were patterned within the EoC along with HUVECs to replicate the patient-specific endometrial environment at the time of biopsy. Of note, endometrial epithelial organoids were utilised for the EoC patterning to reconstruct the patient’s unique endometrial epithelium, preserving the patient-derived expression profiles of vital adhesion molecules, including integrin αvβ3 and OPN, which play a critical role in determining patient’s own endometrial receptivity, aligning with previous findings34 (Fig. 1d). Following these sequential steps, the EoC was allowed for multiple-layer-interactions within each compartment by culturing for an additional six days, which enabled the formation of a 3D cellular distribution that replicates the molecular and cellular environment of the endometrium. In particular, the formation of angiogenic sprouts from endothelial cell channels extended into adjacent endometrial stromal cell channels, replicating the process of neovascularisation that occurs in the endometrial tissue. By incorporating representative fluorescence images, patient-derived vascularised EoCs were successfully displayed (Fig. 1e and Supplementary Movie 1). The EoCs derived from patients who were diagnosed with normal or abnormal endometrial conditions were evaluated by examining the biochemical intensity of endometrial receptivity-related markers and the degree of angiogenesis using angiogenesis-related markers. Based on these findings, we used EoCs for qualitative and physiological evaluations and proposed a unique evaluation system: the Endometrial Receptivity Scoring System—ERS2. By utilising the ERS2 on the EoCs, the status of endometrial receptivity across different patients can be successfully evaluated, providing personalised treatment strategies accordingly (Fig. 1f). The overall process of reconstructing patient-derived vascularised EoC is shown in Fig. 1f.
a Illustration of endometrial architecture during the proliferative and secretory phases of the menstrual cycle, and the early phase of pregnancy, emphasising key events, including angiogenesis and trophoblast invasion at the initial stage of embryo implantation. Created in BioRender. Ahn, J. (2025) https://BioRender.com/bl56qhvb Schematic of the microfluidic chip design incorporating endometrial epithelial organoids, stromal cells, and endothelial cells to replicate in vivo endometrial tissue structure and function. c Step-by-step process for constructing the endometrial environment in the EoC model. d Immunofluorescence images showing expression of EpCAM (i-yellow), integrin αvβ3 (ii-red), OPN (iii-green), and F-actin (iv-white) with DAPI (blue) as nuclear counter-stain in the endometrial epithelial organoids (i-iii) or stromal cells (iv). Scale bar: 200 μ. e Representative stacked confocal images of a patient-derived EoC displaying immunofluorescence images of CD31 (red), F-actin (green), and EpCAM (white) with DAPI (blue). The sectional view displayed by x-z or y-z plane, showing the hollow lumen of the endothelial cell channel pointed with white arrowheads, scale bars: 200 µm. f An overview of the patient-specific EoC model, outlining the isolation of patient-specific endometrial samples, and their integration into the device for personalised evaluation of endometrial receptivity, including the assessment of key endometrial receptivity and angiogenesis markers integrated into the ERS2. Created in BioRender. Ahn, J. (2025) https://BioRender.com/bl56qhv.
Reconstitution of parental tissue-derived endometrial features in the EoCs for modelling trophoblast invasion
We next examined how effectively the developed EoC replicated the estrous cycle of the mouse as it occurs in vivo. To mimic the endometrial environment at each stage of the estrous cycle, which comprises the proestrus (P), estrus (E), metestrus (M), and diestrus (D) phases, in the microfluidic system, endometrial cells were obtained from each estrous stage of the endometrium confirmed through vaginal cytology (Fig. 2a). At each stage, the similarity between the parental tissues and cellular composition displayed in the EoC was examined by evaluating the expression patterns of tissue-specific steroid hormonal receptors, estrogen receptor β subunit (ER-β) (Fig. 2b, c) and progesterone receptor (PR) (Fig. 2d, e), and a representative endometrial receptivity-related marker, OPN (Fig. 2f, g). The ER, which is activated by oestrogen, regulates endometrial proliferation and is typically expressed during the estrus phase35,36. In the epithelial compartment of the EoC, ER-β was highly expressed during the estrus phase compared with those of others, which was consistently shown in the luminal epithelium of mouse endometrial tissues. However, there were no statistically significant differences in the comparisons between the groups (Fig. 2b, c). Moreover, PR, regulated by progesterone, induces changes in the expression of essential cell surface markers and many secretory proteins involved in embryo implantation37. The PR displayed in the stromal channel of the EoC showed a descending pattern in the order of the diestrus, estrus, metestrus, and proestrus, which was consistent with that in the parental tissues (Fig. 2d, e). Furthermore, OPN is among the most consistently identified biomarkers of the receptive endometrium in various species38. OPN is a highly regulated adhesive molecule in the epithelium of the endometrium that becomes receptive to embryo implantation during the estrus phase7. OPN notably showed the highest expression in the EoCs, particularly in the epithelial channel, mimicking the estrus phase, compared with that in devices mimicking other phases of the estrous cycle, which were clearly displayed in the parental tissues (Fig. 2f, g). Moreover, endometrial angiogenesis is a well-known critical component of the receptive endometrium39. Following the estrogen peak prior to ovulation, the most active vascular formation occurs during the estrus phase, providing a richly vascularised endometrium for embryo implantation40. To investigate whether similar endometrial angiogenic activities shown in native tissue were recapitulated in our vascularised EoC, the areas of blood vessels was examined using immunofluorescence for CD31 (Fig. 2h). In our microfluidic system, the areas of angiogenic sprout and blood vessels were significantly increased in the EoC of the estrus phase compared with those of the other stages (Fig. 2h, i). This consistent tendency in the results from the EoCs was validated through comparison with the pattern observed in the native tissue (Fig. 2h, i).
a Schematic of the mouse estrous cycle (proestrus, estrus, metestrus, diestrus), indicating tissue collection points for integration into the EoC. Created in BioRender. Ahn, J. (2025) https://BioRender.com/b09d300b, c Immunofluorescence (IF) images and quantification of ER-β (yellow) with DAPI (blue) in both the EoC (left) and the parental tissues (right) across the estrous cycle (EoC; P vs. D: p = 0.0006). d, e IF and quantification of PR (red) with DAPI (blue) in both the EoC (left) and the parental tissues (right) across the estrous cycle (n = 3 independent mice; one EoC per mouse.) Scale bar: 50 µm. f, g IF of OPN (green) with DAPI (blue) in the EoC and quantification across the estrous cycle (EoC; P vs. E: p < 0.0001, P vs. M: p = 0.0051/ Tissue; P vs. E: p < 0.001, P vs. M: p < 0.0396, E vs. M: p < 0.001, E vs. D: p < 0.001). h, i IF of blood vessel (CD31, red) with DAPI (blue) in the EoC and quantification of blood vessel area (Tissue; P vs. E: p < 0.0013, P vs. M: p < 0.0187, P vs. D: p < 0.0031, E vs. M: p < 0.001, E vs. D: p < 0.001). n = 3 independent mice; one EoC per mouse. Scale bar: 200 µm. j Diagram illustrating trophoblast spheroid aggregation, stromal cell seeding in the EoC, and schematic of the 4-day trophoblast invasion assay. Created in BioRender. Ahn, J. (2025) https://BioRender.com/b09d300k IF images of trophoblast invasion into the stromal layer in the EoC, with F-actin (green), BeWo (red), and DAPI (blue). Scale bar, 50 µm. Quantitative evaluation of the volume of trophoblast spheroid invasion (l), invasion area (m), F-actin volume (n) the number of endometrial stromal cells physically in contact with the trophoblast spheroids (o) over four days (I; D1 vs. D4: p = 0.0031, D2 vs. D4: p = 0.0116/ m; D1 vs. D3: p = 0.0159, D1 vs. D4: p = 0.0008, D2 vs. D3: p = 0.016, D2 vs. D4: p = 0.0008, o; D1 vs. D4: p = 0.0102). n = 3 independent experiments using BeWo spheroids. All data shown as mean ± SD and analysed by one-way ANOVA including P-values (*p < 0.05, **p < 0.01, *** p < 0.001, ****p < 0.0001.) P Proestrus, E Estrus, M Metestrus, D Diestrus. Source data are provided as a Source Data file.
For successful implantation, the embryo must contact the maternal endometrial epithelial layer at an appropriate timing and ensure stable adhesion to the endometrial surface resulting from the successful maternal-foetal cell-cell interaction2. To recapitulate the maternal-foetal interface at the time of embryo implantation using the EoCs, we adopted41 and modified a spheroid-based 3D microfluidic model (Fig. 2j). In this model, to establish a stable attachment environment for the spheroids to reconstructed the endometrium in the EoC, mouse endometrial stromal cells were loaded into 3D microfluidics designed with crown-like posts31. After 4 days, allowing a day for stabilisation, embryo-like spheroids generated from BeWo cells42,43 were introduced via the media channel and assessed daily. The EoCs were fixed and immunostained every other day for four days (Fig. 2k). Over time, trophoblast spheroids exhibited progressive invasion toward the endometrial stromal cells across the epithelial layer, with BeWo spheroids showing a gradual increase in both the volume and area of invasion (Fig. 2l, m). Moreover, even though there were no statistically significant differences, an increasing pattern was observed in the volume of endometrial stromal cells assembled around the trophoblast spheroids, which was determined by measuring the 3D volume of F-actin expression surrounding the BeWo spheroids, displaying more interactions and networks between the two cell types (Fig. 2n). This was corroborated by manually assessing the number of stromal cells directly in contact and interacting with the trophoblast spheroids, which showed a significant increase in the number of endometrial stromal fibroblast cells physically in contact with the trophoblast spheroids over time (Fig. 2o). This can be applied to understand the complex role of the maternal-foetal communication during the initial stage of embryo invasion and early placental development.
Evaluation of patient-specific endometrial environment reconstituted in the EoC using an endometrial receptivity scoring system, ERS2
We next investigated the patients’ endometrial environments through recapitulating their endometrial microenvironments using the EoCs. Patient-derived endometrial epithelial organoids, stromal cells, and HUVECs were patterned within the EoCs and analysed following a six-day culture period (Fig. 3a). To accurately reflect the unique characteristics of each patient’s endometrial environment at the time of biopsy, primary epithelial and stromal cells were isolated from endometrial tissues of twelve independent patients presenting with various subfertility complications, including morphologically and functionally normal endometrium with an embryo or male factor, RIF and/or repeated pregnancy failure (RPL), recruited from the ‘CHA Fertility Centre Bundang’ or ‘Best of ME Fertility Clinic’. All samples were prepared according to each patient’s specific WOI period (Fig. 3b). We established individualised EoCs for each patient, using patient-derived primary epithelial organoids, stromal cells, and HUVECs to assess each patient’s endometrial environment, with particular focus on endometrial receptivity and angiogenesis. To evaluate these parameters, integrin αvβ3 and OPN—markers for endometrial receptivity, and the area of blood vessels and vessel sprouting—standards for endometrial angiogenesis, were used as primary indicators.
a Schematic of experimental design patterning with patient-derived endometrial epithelial organoids and stromal cells in the EoC, replicating the patient’s unique endometrium. Created in BioRender. Ahn, J. (2025) https://BioRender.com/d88wxaub Information of patients whose cells were utilised to pattern within the EoCs. c Immunofluorescence (IF) images of the endometrial receptivity marker, integrin αvβ3 (red) and OPN (green) with DAPI (blue) in patient-derived EoC. Scale bar: 50 µm. Quantification of the intensity of integrin αvβ3 and OPN shown in (c) in a graph of (d) and (e), respectively (d; Normal vs. #B: p = 0.0005, Normal vs. #C: p = 0.0029/ e; Normal vs. #A: p < 0.0001, Normal vs. #C: p < 0.0001). n = 3 independent patient-derived EoCs. f IF images of the blood vessel (CD31; red) with DAPI (blue) in patient-derived EoC. Scale bar: 200 µm. Quantification of the area of angiogenic sprout (g) and blood vessels (h) (g; Normal vs. #A: p < 0.0001, Normal vs. #C: p < 0.0001/ h; Normal vs. #A: p = 0.0019, Normal vs. #B: p = 0.0109). n = 3 independent patient-derived EoCs. Data shown as mean ± SD and analysed by one-way ANOVA including P-values (*p < 0.05, **p < 0.01, *** p < 0.001, ****p < 0.0001). Source data are provided as a Source Data file.
In order to evaluate each patient’s endometrial microenvironments recapitulated within the EoC, we developed the Endometrial Receptivity Scoring System (ERS2) to examine each patient’s endometrial status for the embryo implantation during IVF-ET based on quantifiable values obtained from the EoC, which allows for quick, visual confirmation of results, enabling a more efficient analysis of each patient’s endometrial condition. The ERS2 was established based on four key criteria: intensity of integrin αvβ3 (maximum of 5 points) and OPN (maximum of 5 points), and the area of blood vessels (maximum of 5 points) and angiogenic sprouting (maximum of 5 points), for a total score of 20 points. To establish appropriate scoring ranges for each criterion, we evaluated the ERS2 in six patients (#1-#6) who were clinically diagnosed with a normal endometrium. To establish appropriate scoring ranges for each criterion, we evaluated the ERS2 in six patients (#1–#6) who were clinically diagnosed with a normal endometrium. Four of them (patients #1-#4) were confirmed to have successful pregnancies (Fig. 3b and Supplementary Fig. 1), while the remaining two patients #5-#6) were unable to confirm their pregnancies following the IVF-ET procedure because they were transferred to other clinics (Supplementary Fig. 1). All these patients consistently scored between 3 and 5 points for each criterion (Fig. 3c–h; Patient #1-#3, Supplementary Fig. 1; Patient #4-#6, and Tables 1 and 2). Based on the ranges obtained from these observations, the scoring criteria were defined as follows: a score of 1–2 indicated lower expression relative to the normal range, suggesting insufficiency for achieving a receptive state, and a score > 5 indicated a higher expression than that of the normal range, suggesting over-sufficiency for achieving a receptive state (Table 1). A cumulative score of 12 to 20 was classified within the ‘receptive’ range, suggesting a likely receptive endometrium for embryo implantation during IVF-ET procedures. This was applied to six independent patients with RIF/RPL shown in Fig. 3 (Patient #A-#C) and Supplementary Figs. 1 and 2 (Patient #D-#F).
Our findings indicated that integrin αvβ3 expression was reduced in Patients #B (RIF, 1.3 ± 0.2), #C (RIF, 1.5 ± 0.3), and #E (RIF, 1.6 ± 0.3), all of whom scored 2, compared with those in Patient #1 (Normal, 2.7 ± 0.4) and #5 (Normal, 2.8 ± 0.3), who scored 4, and Patients #2 (Normal, 2.4 ± 0.3), #3 (Normal, 2.2 ± 0.3), #4 (Normal, 2.2 ± 0.3), #6 (Normal, 2.2 ± 0.2), #A (RIF & RPL, 2.1 ± 0.1), #D (RIF, 2.5 ± 0.2), and #F (RIF, 2.6 ± 0.3), all of whom scored 3 (Fig. 3c, d, Supplementary Fig. 1b, c, and Table 2). In contrast, OPN expression was the lowest in Patient #A (RIF & RPL, 1.9 ± 0.1) and #C (RIF, 1.3 ± 0.7), both of whom scored 1, compared with those in Patients #2 (Normal, 6.6 ± 1), #3 (Normal, 5.9 ± 0.2), #B (RIF, 5.9 ± 0.2), #D (RIF, 3.3 ± 0.2), #E (RIF, 4.8 ± 0.4), and #F (RIF, 4.3 ± 0.4), all of whom scored 3, and Patient #1 (Normal, 7.8 ± 0.6), #4 (Normal, 7.5 ± 0.7), #5 (Normal, 7.8 ± 0.6), and #6 (Normal, 7.7 ± 0.3), who scored 4 (Fig. 3c, e, Supplementary Fig. 1b, d, and Table 2). Interestingly, robust OPN expression was observed in both epithelial organoids and stromal cells of Patients #1-#6, who were clinically diagnosed with normal endometrial function. However, in the EoC models of patients #A and #C, both diagnosed with RIF and/or RPL, OPN exhibited a dotted pattern. Notably, in the EoCs recapitulating the endometrium of Patient #B, #E, and #F, OPN expression was observed exclusively in the epithelial cells. Moreover, blood vessels were well-connected with sprouting vessels extending into the stromal cell channel in the EoCs of Patients #1-#6 (Normal-#1;15477 ± 2046, #2;12645 ± 742.7, #3;15492 ± 1025, #4;16996 ± 1514, #5;18576 ± 1177, #6;14733 ± 947.9 µm2, respectively) and #B (RIF-12182 ± 1718 µm2), who were scored 3-4 (Fig. 3f, g, Supplementary Fig. 1b, d, and Table 2). Nevertheless, the EoC of Patient #A (RIF & RPL) frequently exhibited disconnected blood vessels and minimal sprouting of vessels into the adjacent stromal cell channel—features similarly observed in the EoC of Patient #C through #F, with quantified vessel area of 4765 ± 1284 (#C), 3281 ± 778.9 (#D), 3711 ± 622.4 (#E), and 4787 ± 1517 µm2 (#F), resulting in a score of 1 for all (Supplementary Fig. 1e–g). In addition, in the EoCs of Patient #1 ~ #6 (#1;0.4 ± 0.2, #2;0.4 ± 0.03, #3;0.3 ± 0.03, #4;0.4 ± 0.01, #5;0.36 ± 0.01, and #6;0.36 ± 0.06 mm2, respectively), as well as Patients #B (0.4 ± 0.03 mm2), and #D (0.3 ± 0.02 mm2) we observed a blood vessel area larger than that in the EoCs of Patients #A (0.2 ± 0.02 mm2), #C (0.2 ± 0.07 mm2), #E (0.2 ± 0.02 mm2), and #F (0.1 ± 0.03 mm2) (Fig. 3f, h, Supplementary Fig. 1e, g, and Table 2). Applying the ERS2 to the twelve patients (#1-#6 and #A-#F), we found that the EoCs of Patients #1 ~ #6 were totally scored as 13–15, and that of Patient #B had 12 points, considering that their endometrial environments might be receptive and favourable for the embryo implantation. Surprisingly, Patient #B achieved a successful pregnancy without any specific treatments one cycle after the biopsy. In contrast, the EoCs of Patients #A and #C, with scores of 6 and 5, respectively, and those of Patients #D, #E, and #F, with scores 8 to 9, suggested that the endometrial environments in these five patients were insufficiently receptive to embryo implantation in their current state. As expected, it was confirmed that both patients failed to achieve a successful pregnancy in the subsequent IVF-ET cycle (Table 2). All findings of the measurement of endometrial receptivity and angiogenesis observed in the EoCs (Patients #1-#6 and #A-#F) were supported by the marker expressions in parental tissues (Supplementary Fig. 2). These results show that the EoC accurately replicates the patient’s individual endometrial environment, enhancing the understanding of their endometrial conditions.
Following the establishment of the EoC-ERS2 system, which demonstrated functional validity in assessing endometrial receptivity and angiogenesis, we next examined whether the EoC recapitulates the transcriptomic features of patient tissues. QuantSeq 3’ mRNA-seq was performed on patient-derived endometrial tissues and their corresponding EoC samples (normal (n = 2) and RIF (n = 3) for tissues and EoC; total of 10 samples). The analysis identified 43424 differentially expressed genes, of which 1141 were significantly expressed (p < 0.05) within three embryo implantation-related functional categories: endometrial receptivity, hormone responsiveness, and protein secretion/cytokine activity. Among these, 255 genes showed highly similar expression patterns between patient tissues and their paired EoC (Pearson correlation > 0.5). Normalised (log2) expression values were further standardised (each sample to mean signal = 0 and standard deviation = 1) for each sample and visualised as heatmaps (Supplementary Fig. 3a, b). As shown in both heatmaps (without (b-i) and with dendrogram (b-ii)), strong biological concordance was clearly observed between patient tissues and their paired EoC. Differential expression analysis of these 255 genes between normal (n = 2) and RIF (n = 3) groups revealed significant transcriptional differences, with volcano plots highlighting many genes consistently altered across both tissue and chip datasets within the comparison between normal and RIF (red/blue: significant in both, grey: non-significant or data-set specific) (Supplementary Fig. 3c, d). Together, these results demonstrate that the EoC platform faithfully recapitulates endometrial gene expression and captures clinically relevant differences associated with patient conditions.
Category-specific heatmaps further revealed that a substantial subset of genes displayed closely aligned expression profiles between tissues and EoCs, underscoring their high level of biological similarity (Supplementary Fig. 3e). Notably, ITGB3 and SPP1 (encoding OPN)—actively employed in this study as validation markers of endometrial receptivity—were captured within the receptivity category. Their consistent detection across both marker-based and transcriptome-wide analyses reinforces the reliability of the model. To further validate these findings, qRT-PCR was performed on the same patient tissue and paired EoC samples. From the RNA-seq dataset, the top five genes (endometrial receptivity (ARHGAP22, MYH9, CXCL8, BMPER, CPLANE1), hormone responsiveness (MAFA, NLRP3, IL19, PF4, IL24), and protein secretion (VPS54, UBR5, GSTM3, GATA3, YAP1)) showing the highest similarity in expression patterns between the two groups within each functional category were selected, resulting in a total of 15 genes (Supplementary Fig. 4a). In addition, we examined 10 well-established endometrial receptivity-related markers, including SPP1, ITGB3, LIF, MMP2, MMP9, IL1B, TGFBR1, PRLR, IGFBP7, IL11RA (Supplementary Fig. 4b). qRT-PCR confirmed expression trend consistent with those observed in RNA-seq, thereby demonstrating that the EoC faithfully reproduces the molecular features of the patient endometrial microenvironment across key biological dimensions.
Identification of patient-specific therapeutic agents to improve the endometrial microenvironment using the EoC-ERS2
We explored the potential applicability of the ERS2 using the EoC model to improve the diagnostic accuracy for various patient endometrial conditions. To further assess this capability, we recapitulated the endometrium of a patient diagnosed with synechia within the EoC. This approach was designed to identify endometrial condition-specific abnormalities and evaluate therapeutic interventions tailored to the specific needs of patients. EoCs, comprising cells derived from both normal and synechia-affected patients, were subjected to treatment with three distinct pharmacological agents (CXCL1244, human chorionic gonadotropin45; hCG, and vitamin D346; VitD3) and evaluated using the ERS2 after 6 days of treatments (Fig. 4a).
a Schematic of experimental design patterning on the synechia-EoC, with isolated patient-derived cells cultured to replicate the patient’s endometrial microenvironment for drug screening, and the development of the ERS2 for evaluation. Created in BioRender. Ahn, J. (2025) https://BioRender.com/d88wxaub Immunofluorescence (IF) analyses of integrin αvβ3 (red) and OPN (green) with DAPI (Blue) in patient-derived EoCs (normal vs. synechia- NT, +CXCL12, +hCG, or +VitD3) compared to mouse synechia-modelled endometrial tissues under same treatment conditions. Scale bar; 50 µm. Quantification of integrin αvβ3 (c) and OPN (d) IF intensity in the EoCs (c, d) and corresponding mouse endometrial tissues (e, f) (c; Normal vs. NT: p = 0.0293, NT vs. +hCG: p = 0.0004, +CXCL12 vs. +hCG: p = 0.012, +hCG vs. +VitD3: p = 0.0206/ e; Normal vs. NT: p = 0.0024, Normal vs. +CXCL12: p = 0.011, Normal vs. +VitD3: p = 0.0268, NT vs. +hCG: p = 0.012/ f; Normal vs. NT: p = 0.0238, NT vs. +hCG: p = 0.0253) n = 3 independent patient-derived EoCs; n = 3 independent mice. g IF staining images of blood vessels (CD31; red) in patient-derived EoCs (normal vs. synechia- NT, +CXCL12, +hCG, or +VitD3), compared to mouse endometrial tissues under same treatment conditions. Scale bar: 50 µm. Quantification of areas of angiogenic sprout (h) and blood vessels (i) in the EoCs, and blood vessel intensity (j) and blood vessel number (k) in corresponding mouse endometrial tissues (h; NT vs. +CXCL12: p < 0.0001, NT vs. +VitD3: p = 0.0001, +CXCL12 vs. +hCG: p < 0.0001, +hCG vs. +VitD3: p = 0.0001/ i; Normal vs. NT: p = 0.0007, NT vs. +CXCL12: p < 0.0001, NT vs. +VitD3: p < 0.0001, +CXCL12 vs. +hCG: p = 0.0006/ j; NT vs +CXCL12: p = 0.0018, k; Normal vs NT: p = 0.0219, NT vs. +CXCL12: p = 0.0007, NT vs. +hCG: p = 0.0295, NT vs. VitD3: p = 0.0442). n = 3 independent patient-derived EoCs; n = 3 independent mice. Data shown as mean ± SD and analysed by one-way ANOVA including P-values (*p < 0.05, **p < 0.01, *** p < 0.001, ****p < 0.0001.) l IF staining of spheroids loaded into the EoCs (normal vs. synechia- NT or +CXCL12), showing Jeg3 (red), F-actin (green), and DAPI (blue). Scale bar: 50 µm. m Quantification of depth of trophoblast invasion (12 h; Normal vs NT: p < 0.0001, NT vs. +CXCL12: p < 0.0001/ 24 h; Normal vs. NT: p = 0.0103, NT vs. +CXCL12: p = 0.0337/ 48 h; Normal vs. NT: p = 0.0119, NT vs. +CXCL12: p = 0.0104). n = 3 independent experiments using spheroids. Data shown as mean ± SD and analysed by two-way ANOVA including P-values (*p < 0.05, **p < 0.01, *** p < 0.001, ****p < 0.0001). Source data are provided as a Source Data file.
To examine the efficacy of drug treatments on synechia patient-derived EoC, the primary indicators were analysed (Fig. 4b–k). In the EoC of non-treated synechia patient (NT-synechia-EoC), the expression of integrin αvβ3 was significantly lower (0.7 ± 0.3) than that of normal-EoC (2.8 ± 0.4). However, CXCL12 and VitD3 treatments restored integrin αvβ3 levels in synechia patient’s EoC to a level comparable to normal, while hCG treatment substantially improved the therapeutic effects (CXCL12; 2.1 ± 0.5/ hCG; 4.5 ± 1.3/ VitD3; 2.3 ± 0.2) (Fig. 4b, c). Notably, there were no significant differences in OPN expression (Fig. 4b, d). These patterns were consistently observed in mouse endometrial tissue in vivo, where synechia was modelled by physically scratching the uterine wall34, demonstrating the highest therapeutic effect in the hCG-treated group and displaying a restored expressions of endometrial receptivity markers comparable to the levels observed in the normal endometrium (Fig. 4b–f). Subsequently, endometrial angiogenesis was assessed (Fig. 4g–k). In NT-synechia-EoC, blood vessels exhibited poor sprouting (a; 5025 ± 1711 µm2) and a smaller blood vessel area (b; 0.2 ± 0.05 mm2) than that in normal-EoC (a; 15787 ± 2202 µm2 / b; 0.41 ± 0.04 mm2). In contrast, CXCL12-synechia-EoC and VitD3-synechia-EoC demonstrated enhanced blood vessel area (CXCL12; 0.5 ± 0.05 µm2/ VitD3; 0.4 ± 0.07 mm2), with CXCL12-synechia-EoC showing the most significant increase in sprouting vessels (25734 ± 2693 µm2) and blood vessel area (0.5 ± 0.05 mm2) (Fig. 4g–i). Consistent with these findings from the EoCs, CXCL12 treatment induced the greatest increase in blood vessel intensity in the endometrium of the murine model of synechia. Although the total number of vessels was not markedly increased by CXCL12 treatment, full formation of vessels was highly observed likely shown in normal endometrial tissues (Fig. 4g, j, k). Applying these findings to the ERS2, the EoCs-treated with the three drugs achieved scores greater than 12, compared with a score of 5 for NT-synechia-EoC. CXCL12-synechia-EoC achieved the highest score of 16 points, suggesting that CXCL12 was the most effective drug for improving the endometrial environment in this patient (Fig. 4b–k and Table 3). Interestingly, therapeutic efficacy depending on dosages and administration routes was examined for more precise evaluation using the EoC. Two different doses were selected according to previous studies and introduced across all EoCs. This evaluation revealed that all EoCs exhibited distinct responsiveness to each compound depending on dosages (Supplementary Fig. 5a–f). Notably, in the high hCG-treated EoC group, the ERS2 scores were consistently the lowest among all treatment conditions (Supplementary Fig. 5d, e), suggesting that high-dose hCG was relatively ineffective in improving endometrial receptivity within this model composed of endometrial cells obtained from synechia patient. In addition, we further established EoC models in which different drug administration routes (intrauterine vs. intravenous) were applied. Intrauterine administration was mimicked by exposing therapeutic reagents directly to epithelial layer, whereas intravenous administration was mimicked by introducing drugs into media channel directly connected to endothelial channel (Supplementary Fig. 5g). All three therapeutic agents were administered via both routes, and each therapeutic efficacy was evaluated. While no significant differences were observed in the ERS2 for endometrial receptivity between the two administration routes, our findings revealed that CXCL12 was more effectively responded for sprouting area of microvessels via intrauterine administration compared to intravenous route (Supplementary Fig. 5h–m). However, hCG treatment was more effective when administered via intravenous route (Supplementary Fig. 5k–m). Together, these results implicate that the therapeutic efficacy may vary depending on the delivery routes.
The primary objective of enhancing the uterine environment is to optimise the essential conditions necessary for successful embryo implantation. It is hypothesised that improvements in endometrial receptivity and angiogenesis, achieved through drug screening treatment using the EoCs of a synechia patient, may have a positive impact on the embryo implantation process. To develop a more accurate in vitro model for studying the dynamics of embryo implantation, particularly at the maternal-foetal interface, we adopted and modified a spheroid-based 3D microfluidic system (Fig. 4l). This advanced model aims to closely mimic the physiological conditions of the uterine environment, enabling a more detailed exploration of the factors that influence successful embryo implantation. Human endometrial stromal cells were cultured in a 3D microfluidic device with crown-like posts to establish a stable attachment environment for the spheroids. Jeg3 cells exhibit more potent invasive characteristics than other trophoblastic cell lines do, enabling the efficient investigation of the cellular mechanisms underlying trophoblast-endometrium interactions, making them particularly suitable for experiments focused on embryo implantation47. After treatment with CXCL12 in the EoC, identified above, Jeg3-spheroids were introduced through the media channel and observed over 36 h. The spheroids were pre-stained with a cell tracker (GFP) to facilitate live imaging (Supplementary Fig. 6a) and additional sets of the EoCs were fixed for individual immunofluorescence analysis at the indicated time points (Fig. 4l–m). After 2 h of spheroid introduction, the spheroids had already initiated invasion to stromal layer (112.3 ± 3.9) and firmly settled in the normal-EoC (Fig. 4l, m). In contrast, in the NT-synechia-EoC the spheroids failed to settle and floated around inside the channel. After 12 h, the NT-synechia-EoC began to invade at a depth similar to that observed in the normal-EoC at a 2 h time point (117.5 ± 24.1). However, the NT-synechia-EoC (145.4 ± 10.9) showed a significantly lower depth of spheroid invasion than that in the normal-EoC at 36 h of co-culturing (184.1 ± 18.1) (Fig. 4l, m). This finding aligns with the clinical observations of delayed embryo implantation in synechia patients48, suggesting that our EoCs closely replicate individual endometrial characteristics. Nevertheless, both initial attachment and invasion areas were significantly improved by CXCL12 treatment, as shown in the CXCL12-synechia-EoC. When CXCL12 was administered to the NT-synechia-EoC, which showed delayed embryo implantation, the depth of invasion at all time points was similar to that of normal-EoC (CXCL12-2h; 94.2 ± 13.5/ 12 h; 155.5 ± 5.3/ 24 h; 162 ± 8.8/ 36 h;181.9 ± 6.1) (Fig. 4l, m). Moreover, consistent invasion patterns were repeatedly observed in the EoC-spheroid invasion assay using HTR-8/SVneo—a non-tumorigenic, immortalised human extravillous trophoblast cell line widely accepted as a surrogate model for studying trophoblast invasion (Supplementary Fig. 6b). Notably, the differences in invasion depth between synechia-EoC and synechia+CXCL12-EoC was more pronounced in HTR-8/SVneo cells than with Jeg3 cells (Supplementary Fig. 6c), likely reflecting the greater physiological sensitivity of the former to microenvironmental cues. In addition, we introduced Jeg3-spheroids into EoC containing an intact epithelial layer. Interestingly, Jeg3 cells migrated underneath the epithelial barrier and invaded the underlying stromal compartment without visibly disrupting of epithelial integrity (Supplementary Fig. 6d). The depth and pattern of invasion in the presence of the epithelial layer closely resembled that observed in its absence (Supplementary Fig. 6e). This implies that CXCL12, identified as the best treatment for this synechia patient through ERS2, may support the process of embryo implantation and maintain stable attachment and adhesion, suggesting that the EoC-ERS2 is a potent tool for drug screening. Furthermore, to faithfully recapitulate the pre-implantation stage, thereby providing a more physiologically relevant platform for modelling embryo implantation, we incorporated naïve or primed human embryonic stem cells (hESCs) in our EoC platform. Embryoid body (EB) invasion assays using hESCs consistently demonstrated similar invasion patterns under naïve and primed conditions (Supplementary Fig. 7a, b). EBs were generated separately from each state, and their invasion behaviours were analysed within the EoC system. Histological analysis using H&E staining showed that the naïve EBs exhibited cavity structures and clear layer separation (Supplementary Fig. 7c). Gene expression profiling further distinguished the two states, displaying OCT4 was expressed in both, NANOG was enriched in naïve EBs, and OTX2 was upregulated in primed EBs, consistent with their developmental identities49 (Supplementary Fig. 7d–f). These findings confirm that the naïve and primed states were faithfully established in our system. Notably, both naïve and primed EBs exhibited significant differences in invasion depth between EoC-derived from normal and RIF patients, with a more pronounced disparity under the naïve state, likely reflecting the higher trophoblast differentiation capacity of naïve cells relative to the lineage-restricted primed state. In addition, quantitative assessment of OCT4 expression further validated these findings, revealing that OCT4 was preferentially localised toward the stromal compartment and expressed at higher levels in normal EoC compared with the RIF group. Moreover, attachment orientation toward the inner cell mass (ICM) side was clearly identified (Supplementary Fig. 7g–l).
Monitoring progressive improvements in the endometrial receptivity of patients experiencing repeated implantation failure following three consecutive platelet-rich plasma treatments
Four endometrial samples were sequentially collected from a single patient diagnosed with RIF and RPL. Samples were obtained over consecutive menstrual cycles following treatments with PRP at every cycle, which was administered as a clinical intervention aimed at enhancing the endometrial environment, based on the attending clinician’s judgement. The EoC-ERS2 assessment tool was utilised to evaluate the effectiveness of PRP on patient’s endometrium in every cycle (Fig. 5a). PRP treatment is a common therapeutic approach aimed at promoting tissue regeneration and enhancing the rates of embryo implantation. This method leverages growth factors released from concentrated platelets derived from the patient’s own blood, facilitating improved cellular repair and endometrial receptivity50.
a Schematic of experimental design patterning on the EoC with a single RIF/RPL patient-derived endometrial tissue obtained after each clinical PRP administration, and recapitulation of the endometrial environment in the EoC. Created in BioRender. Ahn, J. (2025) https://BioRender.com/d88wxaub Immunofluorescence (IF) staining images of integrin αvβ3 (red) and OPN (green) with DAPI (blue) in patient-derived EoCs compared to parental tissues. Scale bar: 50 µm. c–f Quantification of integrin αvβ3 and OPN IF intensity in the EoCs (c, d) and patient’s endometrial tissue (e, f) (c; Normal vs. T1: p < 0.0001, Normal vs. T2: p < 0.0001, Normal vs. T3: p < 0.0001/ d; Normal vs. T3: p = 0.0387/ f; Normal vs. T1: p = 0.0438). n = 3 independent patient-derived EoCs. Data shown as mean ± SD and analysed by one-way ANOVA including P-values (*p < 0.05, **p < 0.01, *** p < 0.001, ****p < 0.0001). g IF staining of blood vessels (CD31; red). Scale bar: 200 µm. Quantification of area of angiogenic sprout (h) and blood vessel (i) in the EoCs, and blood vessel intensity (j) and blood vessel number (k) in patient’s endometrial tissue (i; Normal vs. T2: p = 0.018, Normal vs. T3: p = 0.0053, T1 vs. T3: p = 0.0184/ k; Normal vs. T1: p = 0.0493, Normal vs. T2: p = 0.0058, Normal vs. T3: p = 0.0015). Data shown as mean ± SD and analysed by one-way ANOVA including P-values (*p < 0.05, **p < 0.01, *** p < 0.001, ****p < 0.0001). Source data are provided as a Source Data file. l The ERS2 of the patient’s endometrium at every treatment. RIF Recurrent Implantation Failure, RPL Recurrent Pregnancy Loss, EMT Endometrial Thickness.
Endometrial tissue from each sample was cultured and utilised to establish the EoC (Fig. 5a). Among the serial EoCs that exhibited the sequential changes in the endometrium as the number of PRP treatments increased, the EoC from the initial sample (NT-EoC), which served as a control and was obtained from the patient before any type of treatments, failed to establish the epithelial layer since epithelial cells from non-treated (NT) control sample were unable to form organoids (Supplementary Fig. 8a, b). The EoCs demonstrated a progressive increase in the intensity of integrin αvβ3 (NT; 0 ± 0/ T1; 1.8 ± 0.2/ T2; 2.3 ± 0.2/ T3; 2.5 ± 0.2) and OPN (NT; 3.1 ± 0.6/ T1; 4.2 ± 1.5/ T2; 4.4 ± 0.6/ T3; 6.1 ± 1.9) expression following repeated PRP treatments (Fig. 5b, c). In particular, OPN expression, which was nearly undetectable in the EoCs of the patient’s endometrium after the first PRP treatment (T1-EoC), appeared as small punctate regions in T2-EoC (after 2nd PRP treatment), and was present almost in all epithelial cells in T3-EoC (after 3rd PRP treatment) (Fig. 5b, d). These expression patterns were consistently observed in corresponding patient tissue samples (Fig. 5b, e, f). Moreover, even though no significant differences were observed in blood vessels sprouting to other channels across all groups, the areas for blood vessels progressively increased with each treatment (NT; 0.2 ± 0.02/ T1; 0.3 ± 0.05/ T2; 0.4 ± 0.02/ T3; 0.4 ± 0.05 mm2) (Fig. 5g–i). Analyses of endometrial angiogenesis depending on the sequential PRP treatments in patient’s tissues revealed an increasing pattern in the intensity of blood vessels, despite no statistically significant differences among groups, and showed that the number of blood vessels formed was increased in the PRP-treated group (NT; 18.0 ± 2.6/ T1; 30.0 ± 6.2/ T2; 31.3 ± 5.5/ T3; 29.3 ± 4.1) (Fig. 5g, j–k). The ERS2 assessment indicated that the endometrial environment showed enhanced receptivity following sequential PRP treatments, with a progressively increasing score from 6 to 16 as the treatments were accumulated (Fig. 5l and Table 4). This improved endometrial environment has been similarly shown in patient endometrial tissue (Supplementary Fig. 8c–f). In patient’s endometrial tissue, Ki-67 expression showed an increasing pattern as PRP treatments were proceeded (Supplementary Fig. 8c–d). Moreover, although it was not statistically significant, a consistent elevation in integrin αvβ3 expression was observed, and the enhanced staining of α-tubulin, an endometrial cilia marker, suggested the regeneration of endometrial epithelial layer after three PRP treatments (Supplementary Fig. 8c, e–f). These findings indicate that the ongoing PRP treatment is proving effective in increasing patient confidence in their current therapy. The ERS2, which quantifies visualised outcomes, allows for an accurate assessment of the current endometrial microenvironment and helps clinicians better determine the optimal timing for embryo implantation during IVF-ET. This enhanced timing precision could ultimately improve pregnancy outcomes, giving patients additional confidence in both the treatment process and their potential for successful embryo implantation. According to the clinical judgement of the attending physician, despite the absence of a visible triple-line of the endometrium, sonographic evaluation consistently revealed no abnormalities, with an endometrial thickness (EMT) exceeding 7 mm in every cycle (Fig. 5a). However, by collecting and analysing the patient’s tissue using the EoC-ERS2, outcomes of embryo implantation at the time of biopsy were predicted by grading the endometrial environment according to established criteria. These changes with sequential PRP treatments included a reduction in predominantly unhealthy cells, restoration of reduced expression levels of endometrial receptivity markers, and recovery of impaired vascular formation (Fig. 5l and Table 4). Conventional ultrasound imaging provides a macroscopic view of the endometrium, whereas the EoC-ERS2 enables the evaluation of the endometrial microenvironment at the cellular and molecular levels, offering a more precise understanding and detailed examination of the endometrium and aiding in determining the optimal timing for embryo transfer to enhance the possibility of successful embryo implantation.
Discussion
Embryo implantation is a pivotal event in establishing a successful pregnancy and relies on three crucial factors, namely a receptive endometrium, a viable and developmentally competent embryo at the blastocyst stage, and a well-synchronised interaction between the maternal and embryonic tissues51. Given the critical role of endometrial receptivity in this process, ET, a cornerstone of ART, particularly in IVF-ET, should be carefully timed to align with the individual period of the WOI52. This procedure involves transferring an embryo, directly into the uterine cavity during a time-sensitive window, which is the optimal period for successful embryo attachment and implantation53. Inadequate evaluation of this parameter can markedly reduce the likelihood of successful implantation. Therefore, identifying and predicting the optimal state of endometrial receptivity is crucial to improving the efficiency of ART and achieving better clinical outcomes.
In this study, we developed a model for evaluating endometrial receptivity by reconstructing the endometrial microenvironment using patient-derived endometrial epithelial organoids, stromal cells, and HUVECs (Fig. 1). Using this model the complex dynamic changes in the endometrial tissue throughout the estrous cycle of mice were effectively simulated, providing valuable insights into the cyclic changes that is governed by fluctuations in sex hormone levels in the endometrium (Fig. 2). Notably, ER expression peaks in the luminal epithelium during the estrus stage, whereas significant PR expression characterises the stromal compartment during the diestrus stage, reflecting a hormonal shift toward progesterone dominance (Fig. 2b–e). Endometrial angiogenesis, a critical determinant of a favourable environment for embryo implantation, is most active during the estrus stage54. In our EoC simulating the estrus stage, we observed substantial vessel formation and development, mirroring the patterns seen in native tissues (Fig. 2h, i). Moreover, the expression of key endometrial markers in the EoC, derived from cells representing different stages of the estrous cycle, is closely paralleled that observed in the parental tissues (Fig. 2f, g). These findings demonstrate that our model successfully recapitulated the dynamic and complex physiological environment of the endometrium across the hormonal stages. Moreover, by incorporating the EoC, we were able to capture crucial maternal-foetal interactions and observe the early stages of embryo implantation (Fig. 2j–o), offering a detailed view of the mechanisms involved in this process. The maternal-foetal interface plays a critical role in the successful implantation. Once the embryo reaches the endometrium, trophoblast cells interact with the endometrial epithelial cells through a network of signalling molecules, including cytokines and growth factors, to facilitate secure attachment to the endometrium55. Following attachment, trophoblasts penetrate through the endometrial epithelium and invade the underlying stromal layer55. Using our EoC, we tracked this invasion process by monitoring the increasing area and volume of trophoblast invasion, as well as the accumulation of stromal cells around trophoblast spheroids (Fig. 2j–o).
One of the primary strengths of our EoC model was its ability to simulate a patient-specific endometrial microenvironment. By utilising the patient samples collected during the same menstrual cycle, defined as the WOI, we have developed a clinically relevant system for assessing the endometrial receptivity, the ERS2 (Fig. 3 and Tables 1, 2). This patient-specific approach is critical, as it enables personalised analysis of endometrial health and the potential for embryo implantation, addressing the individual variations in endometrial biology that may not be captured by traditional diagnostic methods, such as the ERA, a widely used diagnostic tool for evaluating endometrial receptivity through gene expression profiles17. ERA infers tissue conditions with a lack of providing direct observations of protein localisation or functionality56. In contrast, our ERS2 enables real-time visualisation of protein activity, capturing their spatial distribution and functional roles within the tissue. This dynamic view reveals critical biological processes, such as angiogenesis, offering a more detailed understanding of the endometrial environment. Although HUVECs—macrovascular endothelial cells derived from the human umbilical vein—do not fully replicate the uterine milieu, they function effectively as angiogenic biosensors. Their differential responses to patient-specific endometrial conditions and treatments enable comparative scoring. Furthermore, by leveraging the EoC, the ERS2 replicates the physiological characteristics of the endometrium and provides personalised, patient-specific analyses, highlighting its significant potential for clinical applications. In addition, the enhanced efficiency of our platform is demonstrated in its ability to operate with a small number of cells, enabling the generation of numerous patterned chips for a high-throughput analysis. This capability supports the visualisation of protein expression across multiple samples and accelerates the screening of various drug candidates to identify tailored therapeutic strategies that can optimise the implantation process. In the synechia-EoC model, our findings suggested that CXCL12 represents a targeted treatment option for patients with synechia by enhancing blood vessel formation and restoring endometrial function (Fig. 4). This highlights the potential of CXCL12 as a key component of personalised treatment strategies, facilitated by the ERS2, which enables the preclinical assessment of such interventions in a patient-specific context. Furthermore, by assessing the molecular and cellular changes in a patient with a compromised endometrial environment following PRP treatments, we demonstrated its potential to enhance implantation success, highlighting the practical utility of our model not only for assessing receptivity but also for evaluating the efficacy of interventions aimed at improving implantation outcomes (Fig. 5). Although transvaginal ultrasonography performed in the clinic revealed no detectable changes in the patient’s endometrial environment, leading to a diagnosis of unexplained infertility with RIF, our EoC provided critical insights. Following PRP treatment, the patient’s ERS2 significantly improved, indicating a notable enhancement in both the molecular and structural aspects of the endometrium (Fig. 5). After three consecutive PRP treatments, these profound improvements allowed us to predict a successful embryo implantation and pregnancy (Table 4). These findings underscore the utility of the EoC system in delivering real-time, comprehensive assessments of the endometrial microenvironment, thereby facilitating personalised and tailored therapeutic interventions. By uncovering underlying deficiencies that are undetectable through traditional methods and dynamically monitoring treatment outcomes, the EoC demonstrates its transformative potential for precision care in infertility treatment.
Even though the results of our study are promising, it is important to acknowledge that the ERS2 requires further validation using data from larger, diverse populations of healthy patients to establish fully standardised baselines. Comprehensive gene expression analyses, including transcriptomics and hormonal response profiling, would further strengthen the validation of high degree of similarity between the endometrial tissues and the EoC model. Therefore, it might be premature to position the ERS2 as a comprehensive evaluation tool at this stage. Moreover, the current reliance on biomarkers, such as integrin αvβ3, OPN, and angiogenic features, while valuable, may be insufficient to fully capture the multifaceted nature of endometrial physiology. Further research is required to prioritise the identification and integration of additional biomarkers and criteria to enhance the precision and extent of endometrial receptivity assessments. Despite these limitations, the EoC-ERS2 demonstrates significant potential for the diagnosis and treatment of infertility caused by endometrial defects. As additional data from diverse patient populations with larger cohort size are incorporated and new biomarkers are identified, the capacity of the system to deliver precise and comprehensive assessments of endometrial health will be further enhanced. In this study, we initially assessed ERS2 through manual scoring. However, given the inherent limitations of manual immunofluorescence quantification—including labor intensity and inter-observer variability—we developed an automated tool for vascular network morphology analysis, termed Automated Tool for Vascular Network Morphology (ATVNM). To enable systematic and quantitative assessment of vascular architecture, we implemented a comprehensive panel of morphometric indices, including the connected mesh overall parameter (CMOP), debris metrics, mesh number of vessels, largest mesh fraction, weighted thickness uniformity, total area, and total node length. We applied ATVNM to endometrial samples from healthy individuals (Patients #1-#3) and patients with RIP or RPL (Patients #A-#C). Compared to healthy controls, the patient cohort exhibited reduced angiogenic vessel area and length, accompanied by a trend toward fewer vascular branch points and diminished mesh complexity (Supplementary Fig. 9a–k). Notably, Patient #B—the only individual in the patient group to achieve a successful pregnancy—displayed a CMOP distribution within the normal range, suggesting a potential association between preserved vascular network integrity and successful pregnancy outcomes (Supplementary Fig. 9l–v). Ultimately, the EoC-ERS2 has the capacity to become a standardised tool for both basic research and clinical applications, addressing the limitations of current diagnostic methods and enabling the development of personalised and effective treatment strategies.
In summary, we have developed a patient-derived EoC that accurately replicates endometrial receptivity and embryo implantation using primary cells from a patient’s endometrial tissue. This EoC platform offers a comprehensive and personalised approach to studying endometrial receptivity, with significant implications for both diagnostic and therapeutic applications. By simulating the endometrial microenvironment and capturing crucial aspects of implantation, our model provides a valuable tool for understanding the complex biological processes underlying successful pregnancies. Furthermore, its ability to integrate patient-specific data and evaluate the impact of therapeutic interventions provides insights into personalised infertility treatment and precision medicine for reproductive health.
Methods
Microfluidic device design and fabrication
Design and fabrication of microfluidic devices involved the initial preparation of a silicon wafer through photolithography to create a master template. This master template was meticulously engineered to exhibit positive patterns of photoresist, SU-8 (Microchem Laboratory, TX, USA). Subsequently, employing soft lithography and replica molding techniques, the microfluidic devices were constructed utilizing polydimethylsiloxane (PDMS; SYLGARD 184, Dow Corning, Midland, MI, USA). To create the desired mixture, the PDMS elastomer and curing agents were thoroughly blended in a proportion of 10:1 (w/w). This homogeneous blend was poured onto the master mold, followed by a curing process lasting 1 hour within 90 °C dry oven. After completing the curing process, the cured PDMS was delicately removed from the wafer using surgical blade. Utilizing a 1 mm and a 6 mm biopsy punch, cell injection ports and reservoirs for culture media were meticulously created, respectively. It was maintained in 70 °C dry oven after the plasma bonding process for a minimum of 48 h to enhance the hydrophobic nature of the device’s surface. Additionally, before each experiment, the device underwent sterilization by applying UV irradiation, ensuring aseptic conditions.
Human samples
Human endometrial tissue samples were obtained from the patients in the ‘CHA Fertility Centre Bundang’ and ‘Best of ME Fertility Clinic’. Consents were received from every patient after providing them with relevant information. All participants were women aged 30-45 years. This study was allowed by the Institutional Review Board (IRB; approval number 2020–10-007, 1044308-202308-BR-115-01) of the CHA Bundang Medical Centre and CHA University, respectively.
Animals
All animal procedures followed animal care guidelines approved by the CHA University Institutional Animal Care and Use Committee (IACUC; approval number 230062). The young C57BL/6 female mice (6–8-week-old) were purchased from Jabio (Suwon, South Korea). The experimental mice were housed in a specific-pathogen-free (SPF) environment, ensuring controlled lighting conditions (12 h light/12 h dark) and maintaining standard temperature and humidity levels. Before dissecting mouse uteri, vaginal cytology is conducted to identify the different stages of the estrous cycle. The mouse’s tail was gently elevated, and the pipette tip was positioned above the vaginal opening. Using a pipette, 40 μL of saline was gently pipetted 2-3 times. The collected secretion was transferred onto individual glass slides. The slides were placed on a hotplate for drying and stained with 0.1% crystal violet (GC1-OT, BioGNOST, Zagreb, Croatia). Furthermore, images were obtained under a biological microscope (Olympus CKX-53) and four estrous stages were evaluated to the number and appearance of keratinized epithelial cells, nucleated epithelial cells and leucocytes.
Cell culture
Before the experiment, mice were subjected to vaginal cytology smear tests to identify the four estrus cycle stages, followed by harvesting the uteri of mouse for further analyses. The uterine tissue from the sacrificed mice was finely minced using sterile scissors. The tissue fragments were then incubated in an enzyme mixture containing 1.25 IU/mL Dispase II (D4693, Sigma, St. Louis, MO, USA) and 0.4 mg/mL Collagenase V (C9263-100MG, Sigma, St. Louis, MO, USA) at 37 °C for 40 min. For human samples were then incubated in mixture containing 50 μg/mL Liberase (5401119001, Roche, Basel, Switzerland) in Hanks’ Balanced Salt Solution (HBSS, 14170112, Gibco, Grand Island, NY, USA) and 1% BSA at 37 °C for 15 min. The mixture was filtered through a 100 μm (93100, SPL, Pocheon, South Korea) and 40 μm cell strainers (93040, SPL, Pocheon, South Korea) to separate the epithelium and stromal parts. The filtrate was centrifuged at 210 × g for 5 min to remove the supernatant. The resulting pellet was resuspended in Dulbecco’s Modified Eagle Medium/Nutrient Mixture F-12 (DMEM/F12, GIB-11320-033, Gibco, Grand Island, NY, USA) medium supplemented with 20% fetal bovine serum (FBS, 16000-044, Gibco, Grand Island, NY, USA), 1% penicillin-streptomycin (15140-122, Gibco, Grand Island, NY, USA), and 1% L-glutamine (11539876, Gibco, Grand Island, NY, USA). The media were changed every two days. For the experiments, cells from passaging 1-4 were utilised, and subculture was performed when cell confluency reached 70–80% using 0.25% Trypsin-EDTA (CA014-050, GenDEPOT, TX, USA) after washing Dulbecco’s Phosphate-Buffered Saline (DPBS, LB001-02, Welgene, Gyeongsan, South Korea). Human Umbilical Vein Endothelial Cells (HUVECs, CRL-1730, LONZA, Basel, Switzerland) were cultured in endothelial growth medium-2 (EGM-2, CC-3162, LONZA, Basel, Switzerland), and cells from passages 3-5 were used in this experiment.
Generation of endometrial organoid
The epithelial compartment collected from the dissociated tissue was washed with PBS above the cell strainer and centrifuged to form a pellet. The pellet was resuspended in serum-free DMEM/F12 medium and mixed with Matrigel (#354230, Corning, NY, USA) at a 1:10 ratio. The cell-Matrigel mixture was plated in a droplet form in a 48-well culture plate. After incubating for 20 min, organoids maintained in organoids culture medium that consisted of DMEM/F12, 1 X N-2 Supplement (17502-048, Gibco, Grand Island, NY, USA), 1 X B-27 supplement (12587010, Thermo Fisher, Waltham, MA, USA), 1% P/S, 1.25 mM N-Acetyl-L-cysteine (A9165-5G, Sigma, St. Louis, MO, USA), 1 X L-glutamine (25030149, Gibco, Grand Island, NY, USA), 10 M Y27632 (1293823, Biogems, Westlake Village, CA, USA), 10 M A83-01 (9094360, Peprotech, Cedarbrook Dr, USA), 1 mM Nicotinamide (9899208, Biogems, Westlake Village, CA, USA), 50 ng/mL Recombinant human EGF (AF-100-15, Peprotech, Cedarbrook Dr, USA), 100 ng/mL Recombinant human Noggin (120-10 C, Peprotech, Cedarbrook Dr, USA), 500 ng/mL Recombinant human R-spondin1 (120-38, Peprotech, Cedarbrook Dr, USA), 100 ng/mL Recombinant human FGF-10 (100-26, Peprotech, Cedarbrook Dr, USA), 50 ng/mL Recombinant human HGF1 (100-39, Peprotech, Cedarbrook Dr, USA) to at 37 °C under 5% CO257.
Hydrogel and cell loading
Fibrinogen (F8630, Sigma, St. Louis, MO, USA) was dissolved in DPBS and sterilized by filtration (0.22 μm, SLGPR33RS, Millipore). The filtered fibrin gel was combined with aprotinin (0.5 U/mL, A1153, Sigma, St. Louis, MO, USA) in DPBS to produce a fibrinogen solution (Fibrinogen 2.5 mg/mL). Stromal cells (4 × 106 cells/mL) were mixed with the fibrinogen solution and thrombin (0.5 U/mL, T7326-1KU, Sigma, St. Louis, MO, USA) and immediately loaded into the stromal channel. After 1 min at room temperature to solidify the gel, HUVECs (6 × 106 cells/mL) were mixed with the fibrinogen solution and thrombin to assess the blood vessel shape. They were promptly filled into the endothelial channel. Following a 3 min interval, the upper part of the media channel was supplied with EGM-2 medium solution containing sphingosine 1-phosphate (1 nM, Sigma, St. Louis, MO, USA), VEGF-A (100 ng/mL, R&D Systems, Minneapolis, USA), VEGF-C (50 ng/mL, R&D Systems, Minneapolis, USA), and basic fibroblast growth factor (bFGF, 50 ng/mL, R&D Systems, Minneapolis, USA). The bottom part of the media channel was gently aspirated to fill the media channel. After 24 h of incubation at 37 °C, epithelial organoids (4 × 106 cells/mL) formed single cells were condensed into organoid medium and injected into the media channel adjacent to the stromal cell channel. For trophoblast invasion assay, Cell tracker (C34552, Invitrogen, Carlsbad, USA) stained epithelial organoids (4 × 106 cells/mL) injected into the media channel. The device was tilted at a 90° for 40 min to ensure uniformed positioning of the media channel as a monolayer of epithelial organoids on the surface above the stromal cell channel.
Immunofluorescence staining and microscopy
All tissues were fixed with 4% paraformaldehyde (PFA) overnight and, after a day, samples undergone dehydration in 70% ethanol. The tissues were dehydrated using a series of ethanol (80–100%) and cleared using Histoclear (HS-202, National Diagnostics, Charlotte, NC, USA) to remove the ethanol for 2 h. After clearing, the tissues were thoroughly infiltrated with paraffin, they were embedded in molds filled with liquid paraffin and allowed to solidify. After solidification, embedded tissues were cut into 5 μm, dried on glass slides. All tissue samples were deparaffinized using Histoclear. Subsequently, they were dehydrated with a gradient of ethanol ranging from 100% to 80%. Antigen retrieval was performed in a sodium citrate buffer (pH 6.0) at 95 °C for 30 min using a microwave. After washing, specific regions of the tissue samples were restricted using a DAKO pen. Following this, the samples were blocked with 5% bovine serum albumin (BSA) in PBS at room temperature for 1 h. Samples stained overnight at 4 °C using primary antibodies to estrogen receptor (ER, GTX70174, Genetex, Irvine, CA, USA, 1:200), progesterone receptor (PR, #8757, Cell signaling, Danvers, MA, USA, 1:100), Osteopontin (OPN, ab63856, abcam, Cambridge, UK, 1:200), Integrin αvβ3 (ab190147, abcam, Cambridge, UK, 1:100), and CD31 (ab28364, abcam, Cambridge, UK, 1:200). After washing, the samples were subjected to 1 h incubation at room temperature (RT) with secondary antibodies to anti-rabbit IgG fluorescence (Invitrogen, Carlsbad, USA) or anti-mouse IgG fluorescence (Invitrogen, Carlsbad, USA) at a dilution of 1:200. Subsequently, DAPI (62248, Thermo Fisher, Waltham, MA, USA, 1:1000) staining was performed for 10 min. The samples were then mounted with mounting media (S3025, DAKO, Glostrup, Hovedstaden, Denmark) and covered with a cover glass for further analysis. To obtain immunofluorescence staining images in microfluidics, the cells in a microfluidic device were fixed for 15 min with 4% PFA at RT. Next, permeabilization was performed using 0.2% Triton X-100 in PBS for 15 min. Subsequently, the cells were blocked with 3% BSA in Sterilized PBS for 1 h at RT. The samples were incubated overnight at 4 °C with the respective primary antibody to ER (1:200), PR (1:200), OPN (ab63856, ab8448, abcam, Cambridge, UK, 1:200), Integrin αvβ3(sc-7312, santacruz, California, USA, 1:100) and CD31 (130-118-965, Miltenyi, Bergisch-Gladbach, Germany, 1:200) and Phalloidin (A12379, Invitrogen, Carlsbad, USA, 1:400). After a day, the samples were additionally exposed to anti-rabbit IgG fluorescence (A11011, Invitrogen, Carlsbad, USA) or anti-mouse IgG fluorescence (A21235, Invitrogen, Carlsbad, USA) at a dilution of 1:1000, during overnight incubation at 4 °C. The samples were imaged using a confocal microscope (Carl Zeiss ZEISS, Oberkochen, Germany) using the z-stack function, and tile scans were performed when needed. ZEN black edition software (Carl Zeiss, Oberkochen, Germany) was employed for image processing. 3D Confocal video rendering was processed by IMARIS software (Bitplane, Switzerland).
Sample preparation for RNA-Seq analysis
Total RNA from samples used in the experiment was extracted using 500 μl of Labozol (CMRZ001, Cosmo, Seoul, South Korea) per sample. Add 100 μl of chloroform (C2432, Sigma, St. Louis, MO, USA) to the Labozol-containing tube and mix thoroughly. Centrifuge the tube at 15,682 × g for 20 min at 4 °C. The supernatant was transferred to a new tube, an equal volume of isopropanol (33,539, Sigma, St. Louis, MO, USA) was added, and the tube was centrifuged at 15,682 × g at 4 °C for 30 min. The supernatant was discarded, and 1 ml of cold 70% ethanol (100983, MERCK, Darmstadt, Germany) was added to wash the pellet. The tube was centrifuged at 4 °C and 1482 × g for 3 min, and the supernatant was completely removed. A total of 20 μl of ultra-pure water (ML 019-02, Welgene, Gyeongsan, South Korea) was added to the RNA pellet. RNA quality was measured using a NanoDrop spectrophotometer (DeNovix, DS-11FX). RNA with a 260/280 nm absorbance ratio and a 260/230 nm ratio between 1.9 and 2.2 was used for RNA-seq analysis. QuantSeq 3’ mRNA-Seq was performed on paired endometrial tissue and EoC samples from five independent patients (Normal=2, RIF = 3; a total of 10 samples). Raw reads were preprocessed with fastp (v0.23.1) to remove adapters and low-quality sequences. The filtered reads were aligned to the human reference genome (GRCh38) using STAR (v2.7.10b). Gene-level read counts were quantified with featureCounts (v2.0.6). For differential expression analysis, data were normalized using TMM-based log2 (CPM + 1) transformation in the edgeR package (v3.42.4, Bioconductor). Differentially expressed genes (DEGs) were defined with thresholds of p-value (<0.05).
Quantitative real-time PCR analysis
The transcription of 1 μg of RNA into complementary DNA (cDNA) was performed using PrimeScrip™ IV Mix (6215 A, Takara, Shiga, Japan). To evaluate target gene expression, qRT-PCR using SYBR Green (RT500S, Enzynomics, Daejeon, South Korea) was performed on the cDNA. Experimental gene expression data were normalised against the housekeeping gene ACTB. Primer pairs used in the experiments are listed in Supplementary Table 1.
Establishment of a murine synechia model
Synechia mouse models were established as previously described using female mice34. Mice were anesthetized with 2,2,2-Tribromoethanol (Avertin) after selecting those in the estrus stage through vaginal smear testing. To induce a synechia-like mouse models, the syringe needle tip was bent at a 45° angle and inserted into the upper part of the mouse uterus, where it was scraped in all directions to establish a synechia model.
Intrauterine administration of CXCL12, hCG, and VitD3 in murine synechia model
Female mice were anesthetized and modelled for synechia as described previously34,58. After 2 days, mice were randomly divided into 4 groups: Non-treated, CXCL12, hCG, and VitD3. Mice were anesthetized in the previous method, the incision site was re-incised, and CXCL12, hCG, and VitD3 were administered into the uterus. All groups of mice were sacrificed 7 days after treatment to obtain uterine tissue.
Formation of spheroids from trophoblast
The BeWo (CCL-98, human choriocarcinoma cell line, ATCC) and Jeg3 (HTB-36, human choriocarcinoma cell line, ATCC) were obtained and cultured in DMEM/F12 medium supplemented with 10% FBS and 1% penicillin-streptomycin. HTR-8/SVneo were maintained in RPMI1960 media (GIB-11875-093, Gibco, Grand Island, NY, USA) supplemented with 5% FBS (16000-044, Gibco, Grand Island, NY, USA) and 1% penicillin-streptomycin (15140-122, Gibco, Grand Island, NY, USA). Passages were performed when the cells reached approximately 80% confluency using a standard trypsinization method. To generate spheroids, BeWo cells were detached from the culture flasks using 0.25% trypsin-EDTA and resuspended in DMEM/F12 medium. The cell suspension was mixed, then carefully added to ultra-low attachment plates (MS-9096MZ, Sumitomo Bakelite, Tokyo, Japan) containing a total of 250 cells per well and a 1% volume ratio of Matrigel in cultured medium. After 24 h of incubation, a spheroid with a 100–200 μm diameter was used for further analyses.
Human embryonic stem cell (hESC) culture and embryoid body (EB) formation
SNUhES35, SNUhES49, and H9 ESCs were maintained under feeder-free conditions. For SNUhES lines, culture plates were pre-coated with 5 µg/mL vitronectin (VTN-N; Invitrogen, Carlsbad, USA at room temperature for 1 h, and cells were cultured in pre-warmed Essential 8™ medium (Invitrogen, Carlsbad, USA). Colonies were mechanically passaged into small clumps using a modified previously described protocol. Dissociated clumps were evenly distributed onto vitronectin-coated plates, and the medium was replaced daily. For H9 ESCs (human embryonic stem cell line, established at WiCell), culture plates were coated with 0.05% (v/v) hESC-qualified Matrigel (Corning® Matrigel® hESC-Qualified Matrix, #354277, Corning, USA) diluted in DMEM/F-12 with GlutaMAX (10565-018, Gibco, USA) for 1 h at room temperature. After aspirating the coating medium, cells were seeded at 6 × 106 cells/100 mm plate in mTeSR+ medium (StemCell Technologies, ST100-0276) with daily medium changes until reaching ~70% confluency. Cells were detached using ReLeSR (StemCell Technologies, ST058752) according to the manufacturer’s instructions.
Expanded hESC colonies were cut into ~1 mm² fragments using StemPro™ EZ Passage™ (Invitrogen, Carlsbad, USA) and treated with 2 mg/mL collagenase type IV (Invitrogen, Carlsbad, USA) for 10 min at 37 °C until colony edges began to curl and detach. Detached colonies were resuspended in complete EB medium [DMEM/F12 (Invitrogen, Carlsbad, USA), 20% KnockOut™ Serum Replacement (Invitrogen, Carlsbad, USA), MEM Non-Essential Amino Acids (Invitrogen, Carlsbad, USA), and 2-mercaptoethanol (Invitrogen, Carlsbad, USA)] supplemented with 4 ng/mL bFGF (Invitrogen, Carlsbad, USA). Aggregates were cultured in non–tissue culture-treated dishes. After 24 h, hEBs were transferred to fresh non-treated dishes containing EB medium without bFGF.
For H9 ESC-derived EBs, detached ESCs were seeded into 24 well AggreWell 800 plates (34811, StemCell Technologies) pre-treated with Anti-Adherence Rinsing Solution (#07010, StemCell Technologies) and centrifuged at 300 g for 5 min. After rinsing, 1 mL of EB medium (#05893, StemCell Technologies) supplemented with Y-27632 (ab144494, Abcam, UK) was added per well at a density of 1.5 × 106 cells/well, followed by centrifugation at 100 g for 5 min. Aggregates were incubated at 37 °C, 5% CO2. On Day 1, half of the medium was replaced with fresh EB medium, and from Day 2 onward, a conditioned iPSC medium [DMEM/F-12 with GlutaMAX, 20% KnockOut Serum Replacement, 1% MEM NEAA, 100 U/mL penicillin, and 100 µg/mL streptomycin] was used with daily medium changes. EBs were subsequently transferred to ultra-low attachment 96-well U-bottom plates (Corning, #7007, USA) for further culture.
Quantitative morphometric parameters of analysis tools for vascular network morphology (ATVNM)
To objectively and quantitatively evaluate the morphometric features of vascular networks, we developed and utilized a comprehensive suite of analytical indices, detailed below.
Connected mesh overall parameter (CMOP)
The Connected Mesh Overall Parameter (CMOP) integrates multiple conventional morphometric parameters (e.g., connectivity, total vessel area, thickness) into a single quantitative measure to reflect overall vascular network connectivity and structural organization. A higher CMOP value indicates dominance by a larger interconnected vascular structure and suggests a more uniform distribution of vessel thickness across the network.
Debris metrics
Debris Metrics were devised to quantify the degree of vascular fragmentation (debris formation) within networks. We defined vascular debris as isolated vessel fragments or cellular remnants smaller than a specific threshold area (e.g., 1000 pixels). Two parameters were calculated to characterize debris presence quantitatively: the Debris Count (DC), representing the number of debris fragments, and the Debris Fraction (DF), representing the proportion of total vascular area occupied by debris:
A higher debris count and elevated DF imply the prevalence of immature or structurally compromised vessels, reflecting cellular damage or apoptosis leading to fragmented vascular structures.
Mesh number of vessels
The number of black background areas enclosed by blood vessels increases as the vascular network becomes more complex.
The largest mesh fraction
The Largest Mesh Fraction (LMF) parameter quantifies the proportion of the largest single interconnected vascular structure (mesh) relative to the entire vascular network area within an image:
A high LMF value suggests a vascular network dominated by a prominent, thick vessel structure, whereas a low value indicates the presence of numerous smaller, dispersed vascular structures.
Weighted Thickness Uniformity
Weighted Thickness Uniformity (WTU) was introduced to quantify the homogeneity of vessel thickness distribution within vascular networks. WTU is calculated by applying area-weighted thickness averages and standard deviations to assess thickness uniformity across the entire network:
Here, \({{WT}}_{{mean}}\) and \({{WT}}_{{std}}\) are defined as:
WTU values approaching 1 indicate uniform vessel thickness distribution, while values near or below 0 signify considerable thickness heterogeneity within the network.
Total area and total node length
Total Area and Total Node Length represent fundamental morphometric parameters, quantifying total vascular area and the cumulative length of vascular structures, respectively. These parameters enable comparisons across experimental conditions and facilitate analyses of area-length relationships, providing insights into the growth patterns and morphometric trends of the vascular networks.
Statistical analysis
Statistical analyses were conducted to compare different groups using appropriate tests based on data distribution. For parametric distributions, ordinary one-way ANOVA with Dunnett’s multiple comparison test was applied for multiple comparisons. For all analyses, statistical significance was determined at the following levels: P < 0.05 (P < 0.05(*), P < 0.01(**), P < 0.001(***) and P < 0.0001(****)). Prism 6 software (GraphPad Software Inc., La Jolla, CA, USA) was employed for statistical computations.
Ethical statement
All experimental procedures using animals and animal use protocol have been approved by the Animal Ethics Committee of the CHA University (IACUC; approval number 230062). Moreover, written informed consent for the use of human samples and related clinical data was obtained from each patient and this study was approved by the Institutional Review Board (IRB; approval number 2020–10-007, 1044308-202308-BR-115-01) of the CHA Bundang Medical Centre and CHA University, respectively. Naïve-state human embryonic stem cell (hESC) lines SNUhES35 (46,XY) and SNUhES49 (46,XX) were obtained from the Institute of Reproductive Medicine and Population, Seoul National University Medical Research Center. All experiments using Naïve-state hESC lines were reviewed and approved by the Institutional Review Board (IRB) of the Institute of Reproductive Medicine and Population, Seoul National University Medical Research Center (IRB No. 219932-202507-04-01-01). Additionally, H9 ESCs, which is exempt from IRB approval, were kindly gifted by Prof. Jong-Il Kim (Seoul National University).
Data availability
The RNA-seq data generated in this study have been deposited in the Gene Expression Omnibus (GEO) under accession code GSE307510. The authors declare that all other data supporting the findings of this study are available within the article and its Supplementary information files. Source data are provided with this paper.
References
Jabbour, H. N., Kelly, R. W., Fraser, H. M. & Critchley, H. O. Endocrine regulation of menstruation. Endocr. Rev. 27, 17–46 (2006).
Diedrich, K., Fauser, B. C., Devroey, P. & Griesinger, G. The role of the endometrium and embryo in human implantation. Hum. Reprod. Update 13, 365–377 (2007).
Rashid, N. A., Lalitkumar, S., Lalitkumar, P. G. & Gemzell-Danielsson, K. Endometrial receptivity and human embryo implantation. Am. J. Reprod. Immunol. 66, 23–30 (2011).
Aghajanova, L., Hamilton, A. E. & Giudice, L. C. Uterine receptivity to human embryonic implantation: histology, biomarkers, and transcriptomics. Semin Cell Dev. Biol. 19, 204–211 (2008).
Boldeanu, L. et al. The role of mediating factors involved in angiogenesis during implantation. Rom. J. Morphol. Embryol. 61, 665–672 (2020).
Chen, X. et al. Physiological and pathological angiogenesis in endometrium at the time of embryo implantation. Am. J. Reprod. Immunol. 78, https://doi.org/10.1111/aji.12693 (2017).
Kang, Y.-J., Forbes, K., Carver, J. & Aplin, J. D. The role of the osteopontin–integrin αvβ3 interaction at implantation: functional analysis using three different in vitro models. Hum. Reprod. 29, 739–749 (2014).
Hsueh, Y. W. et al. Finding of the optimal preparation and timing of endometrium in frozen-thawed embryo transfer: a literature review of clinical evidence. Front. Endocrinol. 14, 1250847 (2023).
Kim, A., Jung, H., Choi, W. J., Hong, S. N. & Kim, H. Y. Detection of endometrial and subendometrial vasculature on the day of embryo transfer and prediction of pregnancy during fresh in vitro fertilization cycles. Taiwan J. Obstet. Gynecol. 53, 360–365 (2014).
Gao, G. et al. Endometrial thickness and IVF cycle outcomes: a meta-analysis. Reprod. Biomed. Online 40, 124–133 (2020).
Zhao, J., Zhang, Q. & Li, Y. The effect of endometrial thickness and pattern measured by ultrasonography on pregnancy outcomes during IVF-ET cycles. Reprod. Biol. Endocrinol. 10, 100 (2012).
Ruiz-Alonso, M. et al. The endometrial receptivity array for diagnosis and personalized embryo transfer as a treatment for patients with repeated implantation failure. Fertil. Steril. 100, 818–824 (2013).
Maged, A. M. et al. The measurement of endometrial volume and sub-endometrial vascularity to replace the traditional endometrial thickness as predictors of in-vitro fertilization success. Gynecol. Endocrinol. 35, 949–954 (2019).
Bahrami, F., Eftekhar, M. & Zanbagh, L. Uterine artery Doppler and endometrial blood flow in frozen embryo transfer: a cohort study. Int. J. Reprod. Biomed. 21, 205–212 (2023).
Groenewoud, E. R. et al. Influence of endometrial thickness on pregnancy rates in modified natural cycle frozen-thawed embryo transfer. Acta Obstet. Gynecol. Scand. 97, 808–815 (2018).
Jinno, M. et al. Measurement of endometrial tissue blood flow: a novel way to assess uterine receptivity for implantation. Fertil. Steril. 76, 1168–1174 (2001).
Liu, Z. et al. The clinical efficacy of personalized embryo transfer guided by the endometrial receptivity array/analysis on IVF/ICSI outcomes: a systematic review and meta-analysis. Front. Physiol. 13, 841437 (2022).
Haas, J. & Casper, R. F. Observations on clinical assessment of endometrial receptivity. Fertil. Steril. 118, 828–831 (2022).
Sun, B. & Yeh, J. Non-invasive and mechanism-based molecular assessment of endometrial receptivity during the window of implantation: current concepts and future prospective testing directions. Front. Reprod. Health 4, 863173 (2022).
Bhatia, S. N. & Ingber, D. E. Microfluidic organs-on-chips. Nat. Biotechnol. 32, 760–772 (2014).
Galie, P. A. et al. Fluid shear stress threshold regulates angiogenic sprouting. Proc. Natl. Acad. Sci. USA 111, 7968–7973 (2014).
Huh, D. et al. Reconstituting organ-level lung functions on a chip. Science 328, 1662–1668 (2010).
Huh, D. et al. A human disease model of drug toxicity-induced pulmonary edema in a lung-on-a-chip microdevice. Sci. Transl. Med. 4, 159ra147 (2012).
Jain, A. et al. Primary human lung alveolus-on-a-chip model of intravascular thrombosis for assessment of therapeutics. Clin. Pharm. Ther. 103, 332–340 (2018).
Jalili-Firoozinezhad, S. et al. A complex human gut microbiome cultured in an anaerobic intestine-on-a-chip. Nat. Biomed. Eng. 3, 520–531 (2019).
Blundell, C. et al. Placental drug transport-on-a-chip: a microengineered in vitro model of transporter-mediated drug efflux in the human placental barrier. Adv. Healthc Mater. 7, https://doi.org/10.1002/adhm.201700786 (2018).
Long, T. J. et al. Modeling therapeutic antibody-small molecule drug-drug interactions using a three-dimensional perfusable human liver coculture platform. Drug Metab. Dispos. 44, 1940–1948 (2016).
Tsamandouras, N. et al. Quantitative assessment of population variability in hepatic drug metabolism using a perfused three-dimensional human liver microphysiological system. J. Pharm. Exp. Ther. 360, 95–105 (2017).
Zhang, Y. S. et al. Bioprinting 3D microfibrous scaffolds for engineering endothelialized myocardium and heart-on-a-chip. Biomaterials 110, 45–59 (2016).
Kujala, V. J., Pasqualini, F. S., Goss, J. A., Nawroth, J. C. & Parker, K. K. Laminar ventricular myocardium on a microelectrode array-based chip. J. Mater. Chem. B 4, 3534–3543 (2016).
Ahn, J. et al. Three-dimensional microengineered vascularised endometrium-on-a-chip. Hum. Reprod. 36, 2720–2731 (2021).
Ingber, D. E. Human organs-on-chips for disease modelling, drug development and personalized medicine. Nat. Rev. Genet 23, 467–491 (2022).
Xiao, S. et al. A microfluidic culture model of the human reproductive tract and 28-day menstrual cycle. Nat. Commun. 8, 14584 (2017).
Hwang, S. Y. et al. Endometrial organoids: a reservoir of functional mitochondria for uterine repair. Theranostics 14, 954–972 (2024).
Lee, H. R., Kim, T. H. & Choi, K. C. Functions and physiological roles of two types of estrogen receptors, ERα and ERβ, identified by estrogen receptor knockout mouse. Lab Anim. Res. 28, 71–76 (2012).
Trukhacheva, E. et al. Estrogen receptor (ER) beta regulates ERalpha expression in stromal cells derived from ovarian endometriosis. J. Clin. Endocrinol. Metab. 94, 615–622 (2009).
Sharkey, A. M. & Smith, S. K. The endometrium as a cause of implantation failure. Best. Pr. Res Clin. Obstet. Gynaecol. 17, 289–307 (2003).
Griffith, O. W. et al. Embryo implantation evolved from an ancestral inflammatory attachment reaction. Proc. Natl. Acad. Sci. USA 114, E6566–e6575 (2017).
Koo, H. S. et al. Non-invasive Intrauterine Administration of Botulinum Toxin A Enhances Endometrial Angiogenesis and Improves the Rates of Embryo Implantation. Reprod. Sci. 28, 1671–1687 (2021).
Reynolds, L. P. & Redmer, D. A. Angiogenesis in the placenta. Biol. Reprod. 64, 1033–1040 (2001).
Li, X. et al. Three-dimensional culture models of human endometrium for studying trophoblast-endometrium interaction during implantation. Reprod. Biol. Endocrinol. 20, 120 (2022).
Ho, H., Singh, H., Aljofan, M. & Nie, G. A high-throughput in vitro model of human embryo attachment. Fertil. Steril. 97, 974–978 (2012).
Stojanovska, V. et al. Characterization of three-dimensional trophoblast spheroids: an alternative model to study the physiological properties of the placental unit. Cells 11, https://doi.org/10.3390/cells11182884 (2022).
Koo, H. S. et al. CXCL12 enhances pregnancy outcome via improvement of endometrial receptivity in mice. Sci. Rep. 11, 7397 (2021).
Lee, D. et al. Effects of intrauterine human chorionic gonadotropin administration on endometrial receptivity and embryo implantation. Life Sci. 311, 121154 (2022).
Lee, Y. G. et al. The therapeutic effects of vitamin D3 administration on the embryo implantation. Biomed. Pharmacother. 176, 116853 (2024).
Drwal, E., Rak, A. & Gregoraszczuk, E. Co-culture of JEG-3, BeWo and syncBeWo cell lines with adrenal H295R cell line: an alternative model for examining endocrine and metabolic properties of the fetoplacental unit. Cytotechnology 70, 285–297 (2018).
Tuuli, M. G. et al. Uterine synechiae and pregnancy complications. Obstet. Gynecol. 119, 810–814 (2012).
Bi, Y. et al. Cell fate roadmap of human primed-to-naive transition reveals preimplantation cell lineage signatures. Nat. Commun. 13, 3147 (2022).
Wang, X. et al. Therapeutic roles of platelet-rich plasma to restore female reproductive and endocrine dysfunction. Front. Endocrinol. 15, 1374382 (2024).
Egashira, M. & Hirota, Y. Uterine receptivity and embryo-uterine interactions in embryo implantation: lessons from mice. Reprod. Med. Biol. 12, 127–132 (2013).
Patel, J. A., Patel, A. J., Banker, J. M., Shah, S. I. & Banker, M. R. Personalized embryo transfer helps in improving in vitro fertilization/ICSI outcomes in patients with recurrent implantation failure. J. Hum. Reprod. Sci. 12, 59–66 (2019).
Achache, H. & Revel, A. Endometrial receptivity markers, the journey to successful embryo implantation. Hum. Reprod. Update 12, 731–746 (2006).
Kim, M. et al. VEGF-A regulated by progesterone governs uterine angiogenesis and vascular remodelling during pregnancy. EMBO Mol. Med 5, 1415–1430 (2013).
Massimiani, M. et al. Molecular signaling regulating endometrium-blastocyst crosstalk. Int. J. Mol. Sci. 21, https://doi.org/10.3390/ijms21010023 (2019).
Ben Rafael, Z. Endometrial receptivity analysis (ERA) test: an unproven technology. Hum. Reprod. Open 2021, hoab010 (2021).
Turco, M. Y. et al. Long-term, hormone-responsive organoid cultures of human endometrium in a chemically defined medium. Nat. Cell Biol. 19, 568–577 (2017).
Li, B., Zhang, Q., Sun, J. & Lai, D. Human amniotic epithelial cells improve fertility in an intrauterine adhesion mouse model. Stem Cell Res. Ther. 10, 257 (2019).
Acknowledgements
This work was supported by grants from the Ministry of Health & Welfare of South Korea (RS-2025-02214614), the Korean fund for Regenerative Medicine grant funded by the Ministry of Science and ICT, and the Ministry of Health & Welfare (22A0106L1 and RS-2025-02223118), and the National Research Foundation of Korea (RS-2024-00338274) to YJK, and the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number: RS-2024-00406054) and the National Research Foundation of Korea (grant number: RS-2023-00240820) to JA.
Author information
Authors and Affiliations
Contributions
Conceptualization: H.S.K., J.A., Y.J.K., Methodology: G.L., Y.G.L., S.Y.H., D.L., J.L., W.J.Y., Y.Y.K., S.K., J.C.P., Investigation: G.L., Y.G.L., S.Y.H., D.L., H.S.K., Visualisation: G.L., Y.G.L., Funding acquisition: J.A., Y.J.K., Project administration: Y.J.K., Supervision: J.A., Y.J.K., Writing—original draft: G.L., Y.G.L., J.A., Y.J.K., Writing—review and editing: J.A., Y.J.K.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks J Kim, Takahiro Arima, and the other anonymous reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Lee, G., Lee, YG., Koo, H.S. et al. Microengineered patient-derived endometrium-on-a-chip for the evaluation of endometrial receptivity and personalised translational medicine. Nat Commun 16, 10439 (2025). https://doi.org/10.1038/s41467-025-65406-7
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41467-025-65406-7