Abstract
The mechanisms driving immune dysregulation in long COVID disease remain elusive. Here we integrated single-cell multiome data, immunological profiling and functional assays to investigate immune alterations across multiple cohorts. A transcriptional state in circulating monocytes (LC-Mo) was enriched in individuals with mild–moderate acute infection and accompanied by persistent elevations of plasma CCL2, CXCL11 and TNF. LC-Mo showed TGFβ and WNT–β-catenin signaling and correlated with fatigue severity. Protein markers of LC-Mo were increased in individuals with pronounced fatigue or dyspnea, and those with severe respiratory symptoms showed higher LC-Mo expression. Epigenetically, LC-Mo exhibited AP-1- and NF-κB1-driven profibrotic programs. LC-Mo-like macrophages in bronchoalveolar lavage samples from individuals with severe respiratory symptoms displayed a profibrotic profile, and individuals with a high LC-Mo transcriptional state showed impaired interferon responses after stimulation. Collectively, our findings define a pathogenic monocyte transcriptional state linking systemic immune dysfunction to persistent long COVID disease, providing mechanistic insights and potential therapeutic targets.
Similar content being viewed by others
Main
Long COVID affects 10–20% of individuals after severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, with symptoms ranging from mild discomfort to severe, long-lasting impairments such as fatigue, respiratory issues and neurological problems. These symptoms can persist for over 3 years (refs. 1,2,3,4,5), representing a substantial health burden and prompting efforts to better characterize long COVID (LC), including biomarker discovery for improved diagnosis6,7,8,9,10.
LC presents with diverse symptoms reflecting multiorgan system abnormalities11,12,13. The evidence suggests multiple possible causes, including persistence of viral remnants or reactivation of latent viruses7,14,15,16,17. Yet, persistent immune dysregulation is a consistent finding in LC studies10,11,14,16,17,18,19. Although most LC cases follow mild-to-moderate acute illness, many studies do not stratify individuals by acute infection (AI) severity6,7,8, which is crucial because severe cases, especially those treated in the intensive care unit, develop immune changes due to intensive medical interventions20,21. Failing to account for these differences may confound LC-associated molecular signatures, highlighting the importance of refined patient grouping.
To address this gap, we stratified individuals with LC by acute COVID-19 severity to better resolve immune heterogeneity and identify molecular features underlying chronic symptoms. We applied single-cell multiomics profiling of peripheral blood mononuclear cells (PBMCs) and measured plasma cytokines from individuals with LC with fatigue and respiratory symptoms using longitudinal and cross-sectional samples. We identified a distinct circulating CD14⁺ monocyte state associated with LC (‘LC-Mo’), which was enriched in individuals with mild-to-moderate AI. This state coincided with persistent elevation of circulating cytokines, indicating systemic inflammation. In two independent cohorts of individuals with LC with severe respiratory symptoms and abnormal lung function, LC-Mo expression was increased in circulating CD14⁺ monocyte subsets. In bronchoalveolar lavage (BAL) myeloid cells from individuals with severe respiratory symptoms, LC-Mo-like macrophages showed a profibrotic gene expression profile. Functionally, CD14⁺ monocytes from individuals with LC-Mo enrichment showed dysregulated responses to ex vivo stimulation, indicating impaired immune regulation. Together, these findings provide systemic insight into the cellular and molecular basis of LC and highlight potential therapeutic targets.
Results
LC has a distinct transcriptome after mild or moderate disease
Individuals presenting with headache, dyspnea or fatigue to the pneumology outpatient clinic at Hannover Medical School (MHH) were recruited according to the German S1 guidelines22 and the Delphi Consensus Criteria21 for LC (4–12 weeks) and post-COVID-19 syndrome (>12 weeks). These criteria included symptoms persisting beyond the acute phase of SARS-CoV-2 infection or its treatment, new symptoms emerging after recovery and attributed to prior infection or worsening of pre-existing conditions. Because heterogeneity in LC molecular profiles may be shaped by acute disease severity and treatment, we stratified individuals with acute SARS-CoV-2 infection (AI) and LC into those with mild-to-moderate (WHO score of 1–5) AI (AIM and LCAM) and those with severe (WHO scores 6–9) AI (AIS and LCAS).
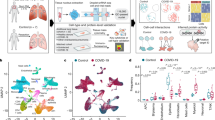
Cohort 1 included 45 individuals recruited between April 2020 and August 2021 at MHH, of which 9 gave longitudinal samples and 36 gave cross-sectional samples (n = 78 total samples), including 11 donors with AI categorized as AIM (n = 7 donors, 42.8% women, median age = 52, range 23–66 years of age, WHO score range 1–5) and AIS (n = 4 donors, 50% women, median age = 37, range 32–54, WHO score range 6–9), 37 donors with LC categorized as LCAM (n = 29 donors, 8 longitudinal donors with two to three time points and 21 single-time-point donors, 58% women, median age = 49, range 31–84 years) and LCAS (n = 8 donors, 3 with two to four time points, 5 single-time-point donors, 25% women, median age = 46, range 19–75) and 8 donors who had recovered after 4–8 months of LC (RLC; 1 longitudinal donor with two time points and 7 single-time-point donors, 37.5% women, median age = 38, range 19–65), in addition to 6 prepandemic noninfected control individuals (NI; 50% women, median age = 40, range 24–61). LC and RLC samples were collected 1.7–10.2 months after infection. Cohort 2 included 117 LCAM donors (24 donors with two to four time points, 93 single-time-point donors, 58.9% women, median age = 48, range 19–83) and 25 LCAS donors (12 longitudinal donors, 13 single-time-point donors, 20% women, median age = 53, range 18–81), recruited between May 2020 and August 2021 at MHH, along with 33 prepandemic NI samples (48.4% women, median age = 40, range 25–65). Cohort 3 included only LCAM donors (n = 8 donors, 62.5% women, median age = 45, range 21–63), all with respiratory postacute sequelae of SARS-CoV-2 infection (PASC) recruited between October and November 2023 at the Pulmonary Rehabilitation Clinic in Schönau am Königssee, Germany. Cohort 4 included LCAM donors (n = 29, 58.6% women, median age = 49, range 33–72), LCAS donors (n = 11 donors, 18% female, median age = 57, range 35–81), 8 donors recovered from AI (RA) and 2 NI donors (60% women, median age = 41, range 29–67) recruited between August 2020 and June 2022 at MHH. Cohort 5 included LC donors (n = 9 donors, 44.4% women, median age = 64, range 62–83, including 5 with respiratory PASC) and NI donors (n = 2 donors, 50% women, median age = 77, range 73–77), recruited between October 2020 and November 2021 at Mayo Clinic, a previously published study23 (Fig. 1a and Methods).
a, Schematic showing the distribution of samples across cohort 1, which included longitudinal and cross-sectional PBMC samples (n = 78) from NI donors (n = 6) and donors with AI (n = 11), LCAM (WHO 1–5; n = 39), LCAS (WHO 6–9; n = 13) and RLC (n = 9), collected 1.7–10.2 months after infection; cohort 2, which included longitudinal and cross-sectional samples (n = 238) from NI donors (n = 33) and donors with LCAM (n = 158) and LCAS (n = 47) collected at 1.5–11 months after infection; cohort 3, which included PBMCs from LCAM donors (n = 8) collected 8–42 months after infection; cohort 4, which included PBMC samples (n = 40) from RA donors and NI donors (n = 10) and donors with LCAM (n = 29) and LCAS (n = 11) collected 3–14 months after infection; and cohort 5, which consisted of PBMC (n = 11) and BAL (n = 9) samples from individuals with LC with unknown acute-phase severity (LCUN; n = 9) and NI donors (n = 2; GEO: GSE263817). b, Number of individuals with LC in cohorts 1–5 exceeding thresholds for fatigue (FAS > 21), respiratory symptoms (dyspnea > 0) or cardiology symptoms (top) and number of samples with pulmonary function tests (PFT), bronchial dilation tests (BDT), blood gas analysis (BGA), electrocardiogram (ECG), FAS and mMRC scores and quality-of-life (QoL) assessments (bottom). Empty boxes denote missing data. c, Distribution of LC samples across months 1.7–2.9(LCAM, n = 10; LCAS, n = 4), 3–5.9 (LCAM, n = 11; LCAS, n = 3), 6–8.9 (LCAM, n = 10; LCAS, n = 4) and 9–11 (LCAM, n = 8; LCAS, n = 2) in cohort 1 (top) and months 1.5–2.9 (LCAM, n = 43; LCAS, n = 8), 3–5.9 (LCAM, n = 56; LCAS, n = 19), 6–8.9 (LCAM, n = 47; LCAS, n = 13) and 9–11 (LCAM, n = 12; LCAS, n = 7) in cohort 2 (bottom). d, UMAP of snRNA-seq data from 78 PBMC samples from all donors and all time points in cohort 1, as in a and c. e, Expression of genes significant by two-sided Wilcoxon test (Benjamini–Hochberg method-adjusted P value of <0.05 and log2 (fold change) > 0.8) in CD14+ monocytes, CD16+ monocytes, CD4+ T cells, CD8+ T cells, B cells and NK cells, with genes consistently upregulated across labeled LCAM time points; HSPC, hematopoietic stem and progenitor cells; moDCs, monocyte-derived dendritic cells; pDCs, plasmacytoid dendritic cells.
Clinical assessment included blood gas analysis, pulmonary function tests and standardized participant-reported outcome measures: the fatigue assessment scale (FAS), validated in chronic fatigue24,25,26 and LC, and the modified medical Research Council (mMRC) dyspnea scale (0–4, where 0 indicates no breathlessness, 1 indicates breathlessness on exertion, 2 indicates breathlessness when hurrying or walking uphill, 3 indicates stopping for breath after ~100 m or a few minutes, and 4 indicates too breathless to leave the house or when dressing), along with quality-of-life metrics27. All clinical assessment data were systematically collected at each participant visit for cohorts 1–4 (Fig. 1b and Supplementary Tables 1–5).
To study molecular signatures of disease progression, we stratified samples in cohorts 1 and 2 by months since AI (months 1.5/1.7–2.9, 3–5.9, 6–8.9 and 9–11; Fig. 1c and Methods). For cohort 1, we generated single-nucleus RNA-sequencing (snRNA-seq) and single-nucleus assay for transposase-accessible chromatin with sequencing (snATAC-seq) data from 78 PBMC samples from NI, RLC, AIM, AIS, LCAM and LCAS donors across all time points. In cohort 2 we measured the concentrations of 14 cytokines in plasma samples from LCAM or LCAS and NI donors across all time points. Validation was performed using single-cell RNA-sequencing (scRNA-seq; cohort 3), flow cytometry (cohort 4) and a published PBMC/BAL single-cell dataset23 (cohort 5). All samples, except those from participants with AI, were PCR negative at collection. We used an integrative multistep analysis to identify cell-type-specific immune dysregulation and link and assess relevance in LC (Extended Data Fig. 1a).
Analysis of single-cell data from cohort 1 PBMCs yielded ~118,000 high-quality cells (Fig. 1d). snRNA-seq data showed distinct patterns in LCAM and LCAS compared to RLC and AI across major PBMCs (Fig. 1e). LCAM showed downregulated AI genes by months 6–8.9, whereas LCAS retained an acute COVID-19-like transcriptomic profile, indicating heterogeneity based on AI history (Fig. 1e). Differential gene expression (DGE) analysis identified 1,737 upregulated genes in CD14+ monocytes from LCAM donors compared to those from AI and RLC (Fig. 1e), with upregulation over 1.7–8.9 months, and showed participant-specific heterogeneity (Fig. 1e and Extended Data Fig. 1b). LCAM CD14+ monocytes showed persistent upregulation of proinflammatory (CSF1, IRF8, RELA and NOTCH1) and anti-inflammatory (TGFB1, SMADs, ENG and SERPINE1) markers (Extended Data Fig. 1c) at all time points, whereas other signature genes showed increased expression from 3 to 8.9 months (Fig. 1e). This signature diminished during months 9–11, possibly due to lower cell numbers (Extended Data Fig. 1d), but showed upregulation of a subset of acute-phase genes, including IL1B, S100A4, PDIA3 and MTRNR2L1. LCAM natural killer (NK) cells also showed distinct increased expression of SREBF1, TAGLN2, TNIP1, NFKBIA and CD83 among others compared to RLC and AI NK cells (Fig. 1e). Collectively, transcriptional profiles in individuals with LC reflected differences based on AI severity, with notable molecular changes in LCAM monocytes and NK cells, whereas LCAS displayed persistent but milder expression of acute-phase genes.
TNF and TNF signaling genes are upregulated in LCAM
We next performed gene set enrichment analysis (GSEA) using pseudobulk counts for each cell subset in LCAM or LCAS samples across all time points, comparing them to the AI and RLC cell samples. LCAM showed persistent upregulation of the TNF signaling pathway and persistent downregulation of interferon (IFN) signaling and response pathways across all major cell subsets (CD4+ and CD8+ T cells, B cells and CD14+ and CD16+ monocytes) compared to AI, up to month 8.9 (Fig. 2a and Extended Data Fig. 2a). CD8+ T cells and NK cells from LCAM samples exhibited increased activation of the ‘TLR signaling cascades’ pathway relative to RLC samples at months 3–8.9 (Fig. 2a). In LCAM CD14+ monocytes, the TNF signaling pathway was transiently upregulated at months 1.7–5.9 and downregulated at months 6–8.9, whereas pathways including transforming growth factor-β (TGFβ), WNT–β-catenin and Notch signaling were upregulated at months 3–8.9 compared to in AI and RLC CD14+ monocytes (Fig. 2a). In LCAS, the TNF signaling pathway was sparsely activated in CD14+ monocytes and CD8+ T cells up to 5.9 months (Extended Data Fig. 2b). LCAS CD14+ monocytes upregulated PD-1 signaling and MHC class II antigen presentation pathways compared to AI, but not RLC (Extended Data Fig. 2b, top). CD8+ and CD4+ T cells and NK cells from LCAS samples displayed increased activation of IFN response pathways compared to CD8+ and CD4+ T cells and NK cells from RLC samples (Extended Data Fig. 2b).
a, GSEA in CD14+ monocytes, CD8+ T cells and NK cells from LCAM samples compared to AI and RLC samples as in Fig. 1e. Pathways are plotted with an adjusted P of <0.1 (Kolmogorov–Smirnov-based test with permutation-derived P values, adjusted using the Benjamini–Hochberg method); NES, normalized enrichment score. b, Expression of CCL2, CXCL11 and TNF in the plasma of NI control individuals (n = 33) and individuals with LC at months 1.5–2.9 (n = 51), 3–5.9 (n = 75), 6–8.9 (n = 60) and 9–12 (n = 19) from cohort 2. Data were analyzed by two-sided Wilcoxon rank-sum test; ****P < 0.00001, ***P < 0.001, **P < 0.01 and *P < 0.05. The box plots show the median (center), first and third quartiles (bounds) and 1.5 times the interquartile range (whiskers). c, Correlation between the amount of TNF in the plasma and pO2 levels in blood in LC donors at months 1.5–2.9 (n = 51), 3–5.9 (n = 75), 6–8.9 (n = 60) and 9–12 (n = 19). Data were analyzed by Spearman correlation. P values were determined using the exact/permutation-based test. The gray shaded area indicates the 95% confidence interval. d, Correlation between AUC score and TGFβ and WNT–β catenin signaling pathways in CD14+ monocytes, IFNα/IFNβ and TLR4 and TLR9 cascade signaling in NK cells and IFNα/IFNβ and WNT–β catenin signaling in CD8+ T cells with a FAS score; red, statistics calculated using only LC samples (LCAM and LCAS); blue, statistics calculated using samples from individuals with LCAM (n = 39), LCAS (n = 13) and RLC (n = 9). Data were analyzed by Spearman correlation. P values were determined using the exact/permutation-based test. The gray shaded area indicates the 95% confidence interval; NS, not significant.
We also profiled 14 proinflammatory cytokines in cohort 2 plasma using a multiplex bead-based assay (Extended Data Fig. 2c), excluding interleukin-4 (IL-4) and IL-5 due to low detection. CXCL11, CCL2 and TNF were persistently elevated in individuals with LC compared to in NI donors up to month 9 (Fig. 2b). TNF mRNA was also persistently upregulated in individuals with LCAM across most immune cell types and time points (Extended Data Fig. 2d). TNF protein exhibited a statistically significant negative correlation with arterial oxygenation (pO2) in individuals with LC (Fig. 2c), which remained statistically significant in LCAM, but not in LCAS, up to month 8.9 (Extended Data Fig. 3a). No other cytokines showed consistent correlations across all time points (Extended Data Fig. 3b,c).
Correlation analysis between key pathways upregulated in CD8+ T cells, NK cells and CD14+ monocytes and FAS scores indicated that TGFβ and WNT–β-catenin signaling in CD14+ monocytes showed modest positive correlations with FAS scores in LC alone and stronger correlations when LC and RLC were combined (Fig. 2d). IFNα/IFNβ induction pathways positively correlated with FAS scores in CD8+ T cells and NK cells in both LC only or LC + RLC combined analyses (Fig. 2d). WNT–β-catenin signaling in CD8+ T cells and Toll-like receptor (TLR) signaling cascades in NK cells, but not TNF signaling in these cells, also correlated with FAS scores (Fig. 2d). These results indicate that persistent upregulation of inflammatory pathways and cytokines in LCAM immune cells might contribute to the clinical symptoms in LC.
The LCAM monocyte signature characterizes a transcriptional state
Next, we performed a reclustering analysis of CD8+ T cells, NK cells and CD14+ monocytes from all donor samples. CD8+ T cells and NK cells each resolved into five clusters (Fig. 3a,b). Differential neighborhood abundance analysis (DA) comparing LC groups at each time point to AI and RLC was performed. A neighborhood defines a small local group of cells with similar gene expression profiles, representing transitional states. LCAM samples exhibited statistically significant increased abundance of neighborhoods in CD69hiCD27hi CD8+ T cells (C3), GZMB+KLRF1+ NK cells (C1) and CD69+TGFB1+ NK cells (C2; Extended Data Fig. 4a,b), whereas LCAS samples showed increased abundance of neighborhoods in C2 NK cells at months 6–8.9 (Extended Data Fig. 4c,d). C3 CD8+ T cells and C2 NK cells showed GZMK+GZMBlo signatures (Extended Data Fig. 4e,f), reported to accumulate after SARS-CoV-2 infection and in aging28,29,30. These clusters showed higher expression of TNF and TLR signaling genes (Fig. 3a,b), suggesting the contribution of persistent TNF signaling in the expansion of CD69hiCD27hiGZMK+ CD8+ T cells and CD69+TGFB1+GZMK+ NK cells in individuals with LCAM.
a, UMAP of CD8+ T cells (left) and violin plots of AUC scores of TNF and TLR1–TLR2 pathways (right) within the identified subclusters CD226+ CD8+ T cells (C0), S100A4+ CD8+ T cells (C1), CD69+GZMK+ CD8+ T cells (C2), CD69hiGZMK+ CD8+ T cells (C3) and KLRC2+KLRD1+ CD8+ T cells (C4) from all donors and all time points in cohort 1, as in Fig. 1a. Data were analyzed by two-sided Wilcoxon rank-sum test; ****P < 0.00001. b, UMAP of NK cells (left) and violin plots of AUC scores of TNF and TLR1–TLR2 pathways (right) in identified subclusters PRF1+GZMB+ NK cells (C0), GZMB+KLRF1+ NK cells (C1), GZMK+TGFB1+ NK cells (C2), IFNG+ NK cells (C3) and CALR+S100A9+ NK cells (C4) from all donors at all time points as in Fig. 1a. Data were analyzed by two-sided Wilcoxon rank-sum test; ****P < 0.00001. c, UMAP of CD14+ monocytes from all cohort 1 donors at all time points showing subclusters IL1B+ (MC1), S100A4+ (MC2), FCN1+ (MC3) and TGFB1+ (MC4) cells. d, Top significantly upregulated markers in MC1–MC4 CD14+ monocyte subclusters as in c. Plotted genes were significant with a Benjamini–Hochberg method-adjusted P value of <0.05 (two-sided Wilcoxon test). e, Differential enrichment of neighborhoods representing transcriptional states in LCAM compared to AIM (top) and LCAM compared to RLC (bottom) at months 1.7–2.9, 3–5.9, 6–8.9 and 9–11. Each dot represents a neighborhood of ~150–400 cells. Transcriptional states show significant enrichment with a spatial false discovery rate (FDR) of <0.1 (F-test statistic from the quasilikelihood F-test, graph-weighted FDR). f, AUC scores of TNF, TGFβ and WNT–β-catenin signaling pathways in MC1–MC4 CD14+ monocyte subclusters as in c. Data were analyzed by two-sided Wilcoxon rank-sum test; ****P < 0.00001. Horizontal dashed lines in a, b and f serve as visual reference for comparison of relative shifts in pathway AUC scores across clusters. g, Correlation of the percentage of MC1, MC2, MC3 or MC4 CD14+ monocyte subclusters with FAS score and pO2 (LCAM, n = 38; LCAS, n = 13; RLC, n = 9). Data were analyzed by Spearman correlation. P values were determined using the exact/permutation-based test. The gray shaded area indicates the 95% confidence interval. h, Box plot showing FAS score in MC4hi (>10% of CD14+ monocytes found within MC4, n = 13), MC4lo (<10% of CD14+ monocytes within MC4, n = 26) and RLC (n = 7) samples from individuals with LCAM, LCAS and RLC. Data were analyzed by two-sided Wilcoxon rank-sum test; **P < 0.01. The box plot shows the median (center), first and third quartiles (bounds) and 1.5 times the interquartile range (whiskers).
Within CD14+ monocytes, four primary clusters (MC1–MC4) were identified (Fig. 3c). MC1 showed high expression of MHC class II molecules, IL1B and NFKB1; MC2 showed elevated NFKB1 and S100A4; MC3 showed increased expression of FCN1, IFN-stimulated genes (IFI44, IFI16 and IFI30) and alarmins S100A8 and S100A9; and MC4 displayed higher levels of IRF1, IRF8, TGFB1, CTNNB1, ENG and NOTCH1, among others (Fig. 3d). DA comparing LC samples with AI and RLC samples across all time points showed a consistent significant increase in MC4 neighborhoods in LCAM in both men and women (Fig. 3e and Extended Data Fig. 4g), with this becoming prominent from month 3 onward (Fig. 3e and Extended Data Fig. 5a). By contrast, MC1 neighborhoods showed a marked increase, primarily at months 1.7–2.9, and ‘tapering off’ by month 11 (Fig. 3e). LCAS did not exhibit consistent changes in MC4, except for a small number of neighborhoods at months 6–8.9 attributable to one participant (Extended Data Fig. 5b). Further, area under the curve (AUC) scores of pathways (calculated per cell from all donors) revealed that MC4, which was uniquely abundant in LCAM, showed significantly higher expression of TGFβ and WNT–β-catenin signaling genes than MC1, MC2 and MC3 (Fig. 3f). MC1 showed higher expression of the TNF signaling genes (Fig. 3f), whereas MC1 and MC3 showed higher IFNγ response gene expression (Extended Data Fig. 5c). We further performed trajectory analysis (unstratified by disease category or groups) that revealed that lineage 3 overlapped closely with the MC4 immune program (Extended Data Fig. 5d,e), indicating that MC4 cells in LCAM have a distinct transcriptional profile compared to MC1–MC3. We next assessed the correlation between the frequency of MC4 within CD14+ monocytes for all LC and RLC samples from all time points with clinical parameters. A modest but statistically significant positive correlation was found between MC4 proportion and FAS score, whereas the correlation with pO2 was negative (Fig. 3g). By contrast, a higher MC1 proportion was negatively correlated with FAS score (Fig. 3g). The modest MC4–FAS correlation likely reflected participant heterogeneity (Extended Data Fig. 5f). Individuals with LC with a high proportion of MC4 (MC4hi) exhibited significantly greater fatigue than those with LC with a low proportion of MC4 (MC4lo) or RLC (Fig. 3h). These findings indicate that increased MC4 abundance (referred to hereafter as LC monocyte transcriptional state (LC-Mo state)) is associated with LC, as demonstrated by its correlation with both FAS scores and pO2 levels.
LC monocytes exhibit increased LC-Mo protein marker expression
To validate the LC-Mo state, we generated and analyzed scRNA-seq data from PBMCs from cohort 3, comprising eight individuals with LCAM with LC symptoms reported for 8–42 months at the time of sampling (Supplementary Table 3 and Methods). All individuals with LC reported fatigue and dyspnea (classified as respiratory PASC (‘Resp-PASC’), n = 5), and three exhibited bronchial hyper-responsiveness (BHR)31, termed ‘Resp-PASC-BHR’ (n = 3). Three clusters (Clust0–Clust2) were identified within CD14+ monocytes (Fig. 4a). Clust1 showed significantly elevated AUC scores for the LC-Mo signature (Fig. 4b). Individuals with Resp-PASC-BHR showed significantly higher expression of the LC-Mo signature in Clust1 than those with Resp-PASC (Fig. 4c), providing independent validation of the LC-Mo state in LCAM and suggesting a link with progression to severe respiratory PASC.
a, UMAP of CD14+ monocytes from individuals with LCAM (n = 8) from cohort 3 showing S100A8+S100A9+ CD14+ monocyte (Clust0), CTNNB1+EMP1+ CD14+ monocyte (Clust1) and FCN1+CCL3+ CD14+ monocyte (Clust2) subclusters. b, LC-Mo signature AUC scores within S100A8+S100A9+ CD14+ monocytes (Clust0), CTNNB1+EMP1+ CD14+ monocytes (Clust1) and FCN1+CCL3+ CD14+ monocytes (Clust2) as in a. Data were analyzed by two-sided Wilcoxon rank-sum test; ****P < 0.00001. c, LC-Mo AUC scores in Clust1 CD14+ monocytes from individuals with LCAM in cohort 3 with Resp-PASC (n = 5) or Resp-PASC-BHR (n = 3). Data were analyzed by two-sided Wilcoxon rank-sum test; ****P < 0.00001. Horizontal dashed lines in b and c serve as visual reference for comparison of relative shifts in pathway AUC scores across clusters. d, Percentage of CD14+ monocytes among PBMCs in donors recovered from acute COVID-19 (RA) combined with NI (RA + NI, n = 10), donors with LCAM (n = 29) and donors with LCAS (n = 11) in cohort 4. e, MFI of HLA-DQ, CD120b, CALR, CD99 and TGFβ in samples from individuals with LCAS, LCAM and RA + NI as in d. f,g, MFI of HLA-DQ, CD120b, CALR and TGFβ in RA + NI (n = 10) and LC donors in cohort 4 categorized based on mMRC dyspnea score (DS) as DS1 (n = 14), DS2 (n = 11) and DS3 (n = 4) (f) or in RA + NI (n = 10) and LC donors in cohort 4 with fatigue (n = 14, FAS score 22–34) and extreme fatigue (n = 14, FAS score 35–47) based on FAS category (g). P values in d–g were calculated using a two-sided Wilcoxon rank-sum test; ****P < 0.00001, ***P < 0.001, **P < 0.01 and *P < 0.05. Box plots show the median (center), first and third quartiles (bounds) and 1.5 times the interquartile range (whiskers).
We next performed flow cytometry analysis on PBMCs from donors in cohort 4, which included 40 LC samples 3–14 months after acute COVID-19 (Supplementary Table 4 and Methods) and 10 RA or NI donors. LC showed a significant increase in CD14+ monocyte percentages compared to RA + NI (Fig. 4d), independent of acute COVID-19 severity. We assessed the expression of 11 proteins (HLA-DR, HLA-DQ, CD105, CD51, TGFβ1, CD99, CD120b, CALR, IRF8, IFNGR1 and CD163) corresponding to LC-Mo transcripts in total CD14+ monocytes in samples from individuals with LC and RA + NI (Extended Data Fig. 6a). Median fluorescence intensity (MFI) of HLA-DQ, CD120b, CALR, CD99 and TGFβ1 was significantly higher in LC compared to in RA + NI (Fig. 4e and Extended Data Fig. 6b), whereas HLA-DR, CD51, CD105, IRF8, IFNGR1 and CD163 showed no significant difference (Extended Data Fig. 6c). Stratification by fatigue scores and dyspnea (range 1–3) revealed consistently higher MFI of CALR, CD120b, HLA-DQ and TGFβ1 in those with more severe LC symptoms (Fig. 4f,g), and TGFβ1 MFI inversely correlated with pO2 (Extended Data Fig. 6d, top). MFI of both TGFβ1 and IRF8 positively correlated with each other (Extended Data Fig. 6d, bottom). Thus, protein markers specific to LC-Mo were elevated in LC, supporting an association between the LC-Mo signature and LC pathology.
Chromatin profiling reveals AP-1/NF-κB1 activity in LC-Mo
We next investigated epigenetic regulation using snATAC-seq data from individuals with LC in cohort 1. Examination of motif signals in the chromatin landscapes of CD14+ monocytes, CD8+ T cells and NK cells from individuals with LCAM compared to those with RLC at multiple time points identified a persistent positive signal for AP-1 family activity in CD8+ T cells and NK cells (Extended Data Fig. 7a). In CD14+ monocytes, AP-1 motif accessibility was elevated up to month 5.9, after which motif enrichment shifted toward transcription factors involved in downstream TGFβ signaling, notably SP1 and KLF family of transcription factors at months 3–8.9 (Extended Data Fig. 7b). MC4 showed the highest number of differentially accessible regions (Fig. 5a). The open chromatin landscape of MC4 showed highest enrichment for motifs for ETS family transcription factors, including GABPA, ETV1, ETV4, SPI1 and SPIC (Fig. 5b). Correlating open chromatin regions with gene expression revealed significant positive associations for proangiogenic and cell adhesion genes (VEGFA, ENG, TGFB1, RXRA, ICAM1 and ITGA5) and genes implicated in inflammatory/metabolic diseases (TTC7A, LMNA and IER3) among others (Fig. 5c). AP-1 family, SMADs, NF-κB1 and RELA transcription factor motifs showed a marked increase within the accessible chromatin regions of these genes (Fig. 5d). Within MC4, correlation of transcription factor transcripts and target gene transcripts with accessible motifs enabled pinpointing of noncoding regulatory regions associated with gene expression, such as those for IER3 and LMNA, and establishment of gene–transcription factor relationships (such as NF-κB1 and AP-1 family likely regulators of LMNA; Fig. 5e–g and Extended Data Fig. 7c). In summary, these findings indicate that LC-Mo is driven by ETS, AP-1 and NF-κB1 transcription factors.
a, Top significant peaks calculated from snATAC-seq data from cohort 1 (n = 78), with aggregated peaks from cells in MC1–MC4 subclusters; data were analyzed by two-sided Wilcoxon test (Benjamini–Hochberg method-adjusted P < 0.05). b, Top ChromVar transcription factor motif enrichment in open chromatin regions in MC4 compared to MC1–MC3; Avg diff, average difference. c, Expression of genes significantly correlated with open chromatin regions within the MC4 subcluster (Pearson’s correlation (Benjamini–Hochberg method-adjusted P < 0.05)). d, Scatter plot of enriched transcription factors motifs (fold enrichment) within open chromatin regions correlated with the expression of genes in c against the ChromVar transcription factors motif enrichment as in b; TF, transcription factor. Dashed horizontal lines represent −0.1 and 0.1 ChromVAR average difference; dashed vertical line represents 0.5 fold enrichment. e, Correlation (Corr) between the expression of genes in c with the expression of transcription factors identified in b and d. Data were analyzed by Pearson’s correlation. Absolute correlations of >0.3 are plotted. The black asterisk indicates transcription factors with motifs in the open chromatin of the correlated gene. f,g, Coverage plots showing the chromatin accessibility regions and gene expression of IER3 (f) and LMNA (g) in subclusters MC1–MC4 and correlations between open chromatin regions and transcription factors with binding sites (gray lined boxes); bp, base pairs; Padj, adjusted P value.
BAL myeloid cells show LC-Mo and profibrotic programs
Circulating monocytes contribute to PASC pathogenesis, particularly pulmonary fibrosis23,32. To assess whether LC-Mo participates in fibrotic lung remodeling, we analyzed paired PBMC and BAL fluid samples from a public dataset23 (cohort 5) consisting of nine individuals with LC of unknown severity during AI (LCUN), classified based on lung function as Resp-PASC (n = 5) or nonResp-PASC (n = 4), and PBMCs from NI donors (n = 2; Supplementary Table 5). Circulating CD14+ myeloid cells were reclustered to identify CD14+CD16− monocytes (Extended Data Fig. 8a), leading to six clusters (CL0–CL5; Fig. 6a). CL5 showed the highest enrichment of LC-Mo signature AUC scores (Fig. 6b and Extended Data Fig. 8b). Within cluster 5, Resp-PASC exhibited significantly higher LC-Mo expression than nonResp-PASC or NI (Fig. 6c).
a, UMAP of CD14+ monocytes cells from PBMCs of cohort 5 (GEO: GSE263817)23 subclusters FABP4+C1QA+ CD14+ monocytes (CL0), NKG7+GZMB+ CD14+ monocytes (CL1), FCN1+S100A9+ CD14+ monocytes (CL2), KLRC2+LAG3+ CD14+ monocytes (CL3), NLRC5+ CD14+ monocytes (CL4) and TREM2+CALR+ CD14+ monocytes (CL5) from NI donors (n = 2) and LCUN donors (n = 9). b, AUC scores of the LC-Mo signature in CL0–CL5 as in a; data were analyzed by two-sided Wilcoxon rank-sum test; ****P < 0.00001. c, AUC scores of the LC-Mo signature in CL5 in PBMC CD14+ monocytes from individuals with LC Resp-PASC (n = 5) and nonResp-PASC (n = 4) and NI donors (n = 2); data were analyzed by two-sided Wilcoxon rank-sum test; ****P < 0.00001. d, Integrated UMAP of CD163+ or CD14+ myeloid cells from PBMCs and BAL samples of individuals with LCUN (n = 9) showing MARCO+FABP4+ macrophages (Cl1), LYZ+CD14+ monocytes (CI2–CI3) and mix clusters from PBMCs and BAL samples with TREM2+CCL2+ (CI4), CCL23+(CI5) and NUPR8+ (CI6). e, UMAP as in d showing CL5 cells. f, LC-Mo AUC score within CI1–CI6 (left) and profibrotic gene signature33 AUC score as in d. Data were analyzed by two-sided Wilcoxon rank-sum test; ****P < 0.00001. Horizontal dashed lines in b, c and f serve as visual reference for comparison of relative shifts in pathway AUC scores across clusters. g, Ratio of CI4/CI5 or CI4/CI6 cells within each individual with Resp-PASC (n = 5) or nonResp-PASC (n = 4). Data were analyzed by two-sided Wilcoxon rank-sum test. Box plots show the median (center), first and third quartiles (bounds) and 1.5 times the interquartile range (whiskers). h, Scatter plots showing log2 (fold change) (log2 (FC)) of DGE in the CI4 versus CI5 and CI4 versus CI6 clusters. Genes significant with an adjusted P value of <0.05 in both comparisons are labeled (two-sided Wilcoxon rank-sum test, Benjamini–Hochberg method-adjusted P < 0.05). All data correspond to cohort 5 scRNA-seq data.
We next integrated CD14+ monocytes from PBMCs and CD163+ or CD14+ myeloid cells from BAL fluid. This integrated dataset identified CI1 with >75% cells from BAL fluid and expressing MARCO+FABP4+, markers for tissue-resident alveolar macrophages, two clusters (CI2 and CI3) with >65% of cells from PBMCs and expressing LYZ+CD14+, markers for circulating monocytes, and three mixed clusters (CI4–CI6) with comparable proportion of cells from both PBMCs and BAL (Fig. 6d and Extended Data Fig. 8c,d). PBMC monocytes in CL5 primarily localized to clusters CI4–CI6 (Fig. 6e), suggesting a macrophage-polarized phenotype. Among these, cluster CI4 had the highest LC-Mo signature enrichment and higher expression of a profibrotic gene set defined in prior COVID-19 BAL studies33 and including TREM2, CALM1, LGMN and APOE (Fig. 6f and Extended Data Fig. 8e). Individuals with resp-PASC showed a higher proportion of CI4 cells and higher CI4/CI5 and CI4/CI6 ratios than individuals without resp-PASC (Fig. 6g and Extended Data Fig. 8f). Differential expression analysis revealed that CI4 cells upregulated the expression of SPP1, CCL13, CCL2 and FOLR2 compared to CI5 or CI6 cells from both individuals with resp-PASC and non-resp-PASC (Fig. 6h). These results indicate LC-Mo enrichment in Resp-PASC PBMCs and its association with a profibrotic transcriptional profile in lung myeloid cells.
Individuals with LC-Mo exhibit dysregulated monocyte function
To assess the functional implications of LC-Mo during immune challenge, we stimulated PBMC samples from cohort 1 (months 1.7–2.9 and 6–8.9) with heat-inactivated Pseudomonas aeruginosa for 4 h and performed single-cell multiome profiling in samples from individuals with LCAM (n = 7), LCAS (n = 5) and RLC (n = 6; Extended Data Fig. 9a,b). Stimulation resulted in a reduction in the numbers of CD14+ and CD16+ monocytes compared to unstimulated samples (Extended Data Fig. 9c,d), consistent with prior reports34,35. Joint analysis of stimulated and unstimulated samples showed that stimulated LCAM CD14+ monocytes exhibited significant downregulation of the inflammatory response, IFNγ signaling, IL-10 signaling, cytokine signaling and IL-6–JAK–STAT3 signaling pathways relative to stimulated RLC CD14+ monocytes (Extended Data Fig. 9e). Next, we classified donors as LC-Mohi (>10% of CD14+ monocytes exhibiting the LC-Mo state) or LC-Molo (<10%); all RLC and LCAS samples were LC-Molo (Extended Data Fig. 5f). Comparison of stimulated LC-Mohi and LC-Molo identified DHFR, HMOX1, EREG and GCLC among the top significantly upregulated DEGs (Fig. 7a). Pathways related to ‘IFNα response’ and ‘cytokine signaling’ were significantly decreased in expression (Fig. 7b) in stimulated LC-Mohi compared to stimulated LC-Molo. At the gene level, stimulation induced cytokine and chemokine gene expression (CCL3, CCL4, CXCL3 and IL6) in both stimulated LC-Mohi and stimulated LC-Molo, whereas IFN response genes (IRF9, ASCC3, XAF1, SAMD9L, LILRB4 and CGAS) were downregulated in LC-Mohi (Fig. 7c). Motif accessibility analysis of stimulated LC-Mohi and stimulated LC-Molo showed that FOXO and TCF (especially TCF7L2) and ZIC motifs were more accessible in stimulated LC-Mohi, whereas stimulated LC-Molo showed increased ETS and AP-1 motif accessibility compared to stimulated LC-Mohi (Extended Data Fig. 9f). Together, these data suggest that LC-Mo might contribute to the functional immune dysregulation observed in individuals with LC.
a, Volcano plot showing DGE in CD14+ monocytes from LC-Mohi (>10% of CD14+ monocytes exhibiting the LC-Mo state) versus LC-Molo (<10% of CD14+ monocytes exhibiting the LC-Mo state) LCAM (n = 7), LCAS (n = 5) and RLC (n = 6) samples collected from cohort 1 at month 1.7–2.9 and month 6–8.9 time points and stimulated with P. aeruginosa for 4 h. Selected significant labeled genes with an adjusted P value of <0.05 are shown in red, whereas all other genes are shown in gray. Data were analyzed by Wilcoxon rank-sum test, Benjamini–Hochberg method-adjusted P < 0.05. b, GSEA enrichment of pathways in nonstimulated LC-Mohi versus LC-Molo and stimulated LC-Mohi versus LC-Molo (unstimulated LC-Mohi, n = 11; unstimulated LC-Molo, n = 23; stimulated LC-Mohi, n = 4; stimulated LC-Molo, n = 14). Pathways plotted with an adjusted P value of <0.1 (Kolmogorov–Smirnov-based test with permutation-derived P values, adjusted using the Benjamini–Hochberg method). c, Dot plot showing gene expression of chemokine and cytokine genes (top) and IFN response genes (bottom) in stimulated and nonstimulated CD14+ monocytes from LC-Mohi and LC-Molo as in a.
Discussion
Using high-resolution single-cell multiome analysis, immunological profiling and functional assays on PBMC and BAL samples from individuals with LC experiencing fatigue and dyspnea, we identified persistent elevations of proinflammatory mediators such as TNF, CCL2 and CXCL11 up to 9 months after infection. We also defined a distinct circulating CD14⁺ monocyte state (LC-Mo) associated with LC. This state, predominant in individuals with LCAM, showed increased TGFβ/WNT–β-catenin signaling that increased over time and exhibited interindividual variability. Individuals with severe resp-PASC displayed higher LC-Mo gene expression, whereas individuals with higher LC-Mo proportions showed reduced IFN responses after in vitro stimulation, suggesting a compromised immune response.
Although 14 individuals in cohort 1 and 51 in cohort 2 were enrolled before the 3-month National Academies of Sciences, Engineering, and Medicine cutoff for LC, over 70% had symptoms extending beyond this period, aligning with established diagnostic criteria. Overlapping symptoms with post-intensive care syndrome complicate LC heterogeneity. Our data revealed molecular differences in PBMCs based on the severity of the AI. Circulating monocytes have been implicated in severe COVID-19 disease and in resp-PASC23,32,33,36,37. Although oxygen saturation in cohort 1 was normal, MC4 cell proportions negatively correlated with oxygen saturation, suggesting subtle gas exchange defects. Nevertheless, increased LC-Mo expression in PBMCs and BAL fluid from individuals with severe resp-PASC in cohorts 3 and 5, together with a profibrotic BAL phenotype, support a link to lung pathology.
Sustained TNF expression, reported in post-COVID cohorts8,13,38, paralleled persistent TNF and/or NF-κB signaling in immune subsets, driving systemic inflammation. Enrichment of GZMK⁺ CD8⁺ T and NK cells in LCAM, shown to expand after SARS-CoV-2 infection30 and in chronic inflammatory diseases28,29,39,40, and increased AP-1 accessibility were consistent with TNF-driven activation41. Upregulated TLR pathways in CD8⁺ T cells and NK cells indicated ongoing viral sensing and potential NF-κB1 activation, fitting with evidence of persistent viral reservoirs or remnants42,43,44,45.
By contrast, CD14⁺ monocytes from individuals with LCAM showed transient TNF signaling, with persistent TGFB1 expression and activation of TGFβ and WNT–β-catenin pathways for up to 11 months. MC4 proportions showed coexpression of TGFβ and IRF8 mRNA and protein, shown to synergistically drive neuroinflammation in the experimental autoimmune encephalomyelitis mouse model46, and displayed motifs for ETS1, AP-1, NF-κB1 and SMAD, transcription factors linked to adhesion and fibrosis47,48. MC4 proportions modestly correlated with FAS scores and blood pO2, and flow cytometry confirmed elevated TGFβ1 in CD14⁺ monocytes. LC-Mo enrichment was also validated in two independent cohorts with severe Resp-PASC patients, thereby linking LC-Mo to lung fibrosis.
Although associations between LC-Mo and symptom severity were noted, correlations were modest, leaving causality undetermined. Further functional studies are needed to clarify these mechanisms. Our study focused on respiratory symptoms and fatigue, so the involvement of LC-Mo in other organ systems remains open. Comparison to unstratified recovered controls (due to limited sample size) restricts interpretation; future work with stratified groups and consideration of vaccination or comorbidities will be important.
In conclusion, we provide a systems view of LC with fatigue and respiratory involvement, identifying a pathogenic monocyte state linked to severe symptoms and offering insights into disease mechanisms and heterogeneity.
Methods
Cohorts
Cohorts 1, 2 and 4
Sample collection and analyses were approved by the ethics committee of the MHH (ethics vote 9001_BO_K). All participants gave written broad consent. Blood was collected from individuals who were admitted to the hospital due to COVID-19, as well as from ambulatory individuals after SARS-COV-2 infection showing symptoms indicative of LC >4 weeks after infection in accordance with the German S1 guideline for LC and post-COVID syndrome at the MHH. Cohort 1 included individuals with AIM (n = 7, 42.8% women, age median = 52, range 23–66 years) and AIS (n = 4, 50% women, age median = 37, range 32–54 years), individuals with LC symptoms (LCAM (n = 29, 8 longitudinal with two to three time points, 21 single-time-point donors, 58% women, median age = 49, range 31–84) and LCAS (n = 8, 3 longitudinal with two to four time points, 5 single-time-point donors, 25% women, median age = 46, range 19–75)), donors recovered from LC (4–8 months of LC; RLC (n = 8, 37.5% women, median age = 38, range 19–65)) and prepandemic NI control individuals (n = 6, 50% women, median age = 40, range 24–61). LC and RLC samples were collected 1.7–10.2 months after infection, and all groups were recruited between April 2020 and August 2021 at MHH. Cohort 2 included individuals with LCAM (n = 117, 24 longitudinal with two to four time points, 93 single-time-point donors, 58.9% women, median age = 48, range 19–83) and LCAS (n = 25, 12 longitudinal, 13 single-time-point donors, 20% women, median age = 53, range 18–81), recruited between May 2020 and August 2021 at MHH, along with prepandemic NI samples (n = 33, 48.4% women, median age = 40, range 25–65). Cohort 4 included individuals with LCAM (n = 29, 58.6% women, median age = 49, range 33–72) and LCAS (n = 11, 18% women, median age = 57, range 35–81) and individuals recovered from AI (RA n = 8 and NI n = 2, 60% women, median age = 41, range 29–67), recruited between August 2020 and June 2022 at MHH. All individuals with AI had a positive SARS-CoV-2 PCR test at admission or externally before admission. All individuals with LC had a prior proven SARS-CoV-2 infection. The SARS-CoV-2 strain in individuals with AI or LC was not recorded. Clinical parameters, including blood gas measurements, pulmonary function tests, FAS and mMRC scores and quality-of-life assessments, were systematically collected at each visit.
Cohort 3
Sample collection and analyses were approved by the ethics committee of the Philipps University Marburg (Az.:24-289 ‘Entschlüsselung der molekularen Pathophysiologie des Post-Covid-Syndroms und prädisponierender Faktoren mit Hilfe neuer Sequenzierungstechnologien und Phänotypisierung von Immunzellen’). All participants gave written broad consent. Samples was collected from individuals with LCAM (n = 8, 62.5% women, median age = 45, range 21–63), all with resp-PASC and recruited between October 2023 and November 2023 during their stay at the Pulmonary Rehabilitation Clinic in Schönau am Königssee, Germany. All individuals with LC had prior proven SARS-CoV-2 infection, and samples were collected >6 months after SARS-CoV-2 infection, one sample per participant. The SARS-CoV-2 strain in individuals with AI or LC was not recorded. Clinical parameters, including blood gas measurements, pulmonary function tests, FAS and mMRC scores and quality-of-life assessments, were systematically collected for each participant.
Cohort 5
Biosample collection for both PBMCs and BAL fluid is available at ref. 23 and included individuals with LC (n = 9, 44.4% women, median age = 64, range 62–83), including five with resp-PASC, and NI donors (n = 2, 50% women, median age 77, range 73–77), recruited between October 2020 and November 2021 at Mayo Clinic.
Sample processing for PBMCs
Sample processing for cohorts 1–4 and storage was performed following the standard procedures of the Hannover Unified Biobank (HUB) as described by Kopfnagel et al.49. PBMCs were isolated from whole blood using Ficoll gradient centrifugation. Cohort 5 PBMC and BAL sample processing was performed similar to as described previously23.
Cytokine assay (cohort 2)
The Quanterix HD SP-X Imaging and Analysis System was used to measure plasma samples. The following panels were used in this study: Human Corplex cytokine panel 1 10-Plex array including IL-12p70, IL-1β, IL-4, IL-5, IL-6, IL-8, TNF, IFNγ, IL-10 and IL-22. The Simoa chemokine panel 1 4-plex kit contained four chemokines, including IP-10 (CXCL10), MCP1 (CCL2), MIP1-β (CCL19) and ITAC (CXCL11). IL-4 and IL-5 were excluded from further analysis due to being below the limit of detection. The detection values were log2 transformed. All plasma samples were processed according to standard biobanking protocols and stored at a minimum temperature of –80 °C. For the experiments, the samples were randomized and measured according to the manufacturer’s manual. The study protocol conformed to the ethical guidelines of the Declaration of Helsinki, and the ethics committee of MHH approved this study a priori (9001_BO_K, No. 9472_BO_K_2020, broad consent: 2923-2015). Informed consent was obtained from all participants included in the study.
P. aeruginosa stock production
A P. aeruginosa clinical isolate CH5464 was streaked from frozen glycerol stocks onto LB agar plates and incubated overnight at 37 °C. Bacteria from single colonies were used to inoculate an overnight preculture in LB medium. This preculture was then diluted in fresh LB medium and grown at 37 °C with shaking at 180 rpm until reaching the early stationary phase. The culture was centrifuged at 10,000g for 10 min, and the supernatant was discarded. The pellet was washed twice with PBS and incubated at 80 °C for 60 min to inactivate the bacteria in a waterbath. Afterward, the suspension was centrifuged again at 10,000g for 10 min to remove cellular debris. The bacterial suspension was adjusted to a concentration of 108 colony-forming units (c.f.u.) per ml and stored at −20 °C for future use. To confirm complete bacterial inactivation, 100 µl of the bacterial suspension was plated on blood agar plates.
In vitro PBMC stimulation
We conducted scMultiome-seq analysis on PBMCs from individuals with LC across five time points: the acute phase; 3, 9 and 12 months after infection and during recovery. Heat-inactivated P. aeruginosa strain and a mock stimulation condition were tested over the course of four experimental runs. A pilot study was performed using samples from two healthy individuals, with cells stimulated for 4 and 24 h at four different concentrations to determine the optimal conditions. Based on this pilot study, the 4-h time point and 2.5 × 106 c.f.u. per ml were identified as optimal.
For the main experiment, PBMCs were thawed according to an optimized protocol based on 10x Genomics guidelines (CG000365, Rev B). The cells were counted and resuspended at a concentration of 5 × 106 cells per ml in warm RPMI. Cell suspension (100 µl) was plated into a 96-well, round-bottom plate and rested for 1 h at 37 °C. Following this rest period, the RPMI medium was replaced with 100 µl of heat-inactivated P. aeruginosa corresponding to a concentration of 2.5 × 106 c.f.u. per ml. The cells were incubated at 37 °C for 4 h. After incubation, the plates were centrifuged at 300g for 5 min, and the cells were collected for nuclei isolation and library preparation.
Isolation of nuclei and library preparation (cohort 1)
scMultiome-seq analysis was performed on both directly thawed and stimulated PBMCs. To manage sequencing costs, cells from three to four donors were pooled together. After pooling, the cells were treated with DNase I to remove free DNA and centrifuged at 300g for 10 min at 4 °C. The cell pellets were resuspended and incubated with 300 µl of prechilled 1× lysis buffer on ice for 3 min. Lysis was stopped by adding 1 ml of ice-cold wash buffer, followed by centrifugation at 500g for 5 min at 4 °C. The nuclei were washed twice with 500 µl of wash buffer and resuspended in Diluted Nuclei Buffer. To ensure purity and dissociation of single nuclei, the suspension was passed through a 40-µm Flowmi strainer and inspected under a microscope.
Approximately 20,000 nuclei were loaded into a Chromium Controller to produce single-cell gel beads, following the 10x Genomics Chromium Next GEM Single Cell Multiome ATAC + Gene Expression protocol (CG000338, Rev C). After transposition, the nuclei were treated with a transposase enzyme, which selectively fragmented the accessible DNA regions and added adapter sequences to the fragmented DNA ends. The transposed nuclei were loaded onto a Chromium Next GEM Chip J (PN-1000234), alongside partitioning oil and barcoded gel beads. PCR amplification was performed, targeting approximately 10,000 nuclei per library. Sequencing was performed using the Illumina NovaSeq 6000 platform, with a minimum read depth of 20,000 read pairs per cell for scRNA libraries and 25,000 read pairs per cell for scATAC libraries.
Cell capture and library preparation (cohort 3)
We thawed cells following the 10x Genomics thawing protocol (CG00039, Rev D), cells from four donors were pooled together, and approximately 29,000 cells were loaded into the Chromium X (10x Genomics) to generate single-cell gel beads in emulsion according to the 10x Genomics protocol (CG000731, Rev A). scRNA-seq libraries were prepared using a Chromium GEM-X Single Cell 3ʹ Reagent Kits v4 (10x Genomics) and sequenced on the NovaSeq 6000 platform (Illumina), with a minimum depth of 20,000 read pairs per cell.
Sample preparation for ex vivo flow cytometry experiments (cohort 4)
PBMCs were isolated from fresh whole blood using standard Ficoll density gradient centrifugation and cryopreserved in liquid nitrogen for deferred use. Ex vivo phenotyping of immune cells was performed from cryopreserved PBMCs. In brief, thawed PBMCs were stained with a Zombie NIR Fixable Viability kit (Biolegend, 423106) at room temperature in PBS for 15 min. Nonspecific immunolabeling conferred by Fc receptor binding was blocked by the addition of 10% Gamunex solution (Grifols Deutschland). Surface marker immunolabeling was performed in cell staining buffer (PBS, BSA and EDTA) (Biolegend, 420201) and Brilliant Stain Buffer (BD, 563794) overnight at 4 °C with antibodies to the ontogeny markers anti-human CD3, CD14, CD16 and HLA-DR; macrophage markers CD163 and CD206 and the markers identified from the transcriptomic analysis CD51, CD99, CD105, CD120b and HLA-DQ (see antibody details in the table). After fixation and permeabilization (BD, 554714) for 30 min at room temperature, immunolabeling of intracellular markers was performed for 30 min in Permwash buffer (BD, 554714) at 4 °C with antibodies to CALR, IFNGR1, TGFB1 and IRF8. Next, cells were immunolabeled with the secondary antibodies AF488 and AF568 for 30 min in Permwash buffer (BD, 554714) at 4 °C to label the unconjugated antibodies CALR and IFNGR1, respectively. All donors were also immunolabeled with the correspondent isotype controls for the used antibodies. Cells were washed with PBS, and data were acquired on a five-laser Sony spectral analyzer (ID7000, Sony) and analyzed with FlowJo software v10.10.0 (Tree Star).
Antibody list
Antibody | Fluorochrome | Clone | Company | Catalog |
|---|---|---|---|---|
CD3 | SparkBlue | SK7 | BioLegend | 344852 |
CD14 | PacBlue | 63D3 | BioLegend | 367122 |
CD16 | BUV563 | 3G8 | BD | 568289 |
CD51 | APC | NKI-M9 | BioLegend | 327913 |
CD99 | PE | hec2 | BioLegend | 398205 |
CD105 | BUV421 | 43A3 | BioLegend | 323219 |
CD120b | PE-DAZZLE | 3G702 | BioLegend | 358413 |
CD163 | FITC/PE-Cy7 | GHI/61 | BioLegend | 333618/2268070 |
CD206 | APC-Cy7/PE-Cy7 | 15-2 | BioLegend | 321120/321124 |
CALR | Purified | Abcam | ab2907 | |
AF488 | Invitrogen | |||
HLA-DQ | BB700 | Tu169 | BD | 745976 |
HLA-DR | AF700 | L243 | BioLegend | 307626 |
IFNGR1 | Purified | Abcam | ab154400 | |
AF568 | Invitrogen | |||
IRF8 | PE | REA516 | Miltenyi | 130-122-927 |
TGFB1 | PE-CF594 | TW4-9E7 | BD | 562422 |
Statistical methods
No statistical method was used to predetermine sample size. The samples were randomized before processing for single-cell experiments. The investigators were not blinded to allocation during the experiments or during outcome assessment. All statistics in the manuscript are reported as specified in the figure legends.
Genotyping
Genotyping of DNA samples isolated from participants in the current study was performed using the GSA-MDv3 array (Infinium, Illumina) following the manufacturer’s instructions. In total, 725,875 variants of 48 individuals were called by Optical 7.0 with default settings
Genotype processing for demultiplexing
Genotype data were reformatted into PLINK binary format files50. Quality control was performed at both the sample and single-nucleotide polymorphism (SNP) levels. Samples were excluded if they exhibited sex mismatches, missing genotyping rates of ≥0.05, heterozygosity rates beyond three standard deviations from the mean or relatedness across samples. A total of 58 samples passed these filters. SNPs were further filtered based on a minor allele frequency of >0.01 and an SNP missingness rate of <0.05. Genotype imputation was conducted using the Minimac4 server51, using the TOPMed r3 reference panel52 and EAGLE v2.4 for phasing. The final analysis included a total of 6,050,031 variants.
Data preprocessing for multiome datasets and demultiplexing
BCL files from each library were converted to FASTQ files using cellranger-arc mkfastq with default parameters and using the respective sample sheet with the 10x barcodes. The 10x Genomics cellranger-arc count pipeline (v2.0.2) was used with default parameters using the human reference genome GRCh38-2020-A-2.0.0 obtained from 10x Genomics website. Demultiplexing was performed using Souporcell (v2.4)53. To assess the concordance between the genotypes of each donor in Souporcell-generated VCF and a reference VCF, BCFtools was used to perform a genotype check with parameter ‘gtcheck’ and the ‘-u GT’ option to compare the genotype fields in the two VCF files.
Quality control and integration of multiome datasets (cohort 1)
Once the donor for each cell was assigned after demultiplexing, only single cells with both RNA and ATAC data were considered. Seurat version 5.0 (ref. 54) was used for downstream analysis. The following filtration criteria were used: ‘nCount_RNA < 6,000 and nCount_ATAC < 15,000, mitochondrial percentage < 20, RNA features < 3,000, TSS enrichment > 1 and <10, while nucleosome_signal < 2’. RNA integration across libraries was performed using ‘RPCAIntegration’ and the top 30 dimensions for both clustering and UMAP generation. Further, multiple resolutions varying from 0.2 to 0.8 were performed to get clusters, and canonical markers were used for identifying cell subsets (using a combination of known markers and those used by Azimuth celltype. l2). For ATAC integration across libraries, signac version 1.13 (ref. 55) was used. Integration anchors were found by using ‘rlsi’ and 2–30 top dimensions, followed by integration using the top 30 dimensions.
Participant and sample stratification and sample category classifications (cohorts 1, 2 and 3)
We first stratified individuals with LC into two groups based on their acute COVID-19 disease WHO scores, where LC samples from individuals with acute COVID-19 WHO scores between 1 and 5 were classified as LCAM and LC samples from individuals with acute COVID-19 WHO scores between 6 and 9 were classified as LCAS. Further samples were also stratified based on time points of collection resulting in NI, AI, T2: 1.5/1.7–2.9 months, T3: 3–5.9 months, T4: 6–8.9 months and T5: 9–12 months.
For cohort 1, to ensure that our findings were not convoluted by COVID-19 infection imprinting on immune cells and were unique to LC, we performed transcriptome comparisons either to AI samples or to RLC. All recovered samples were considered as one category. Consequently, within each group of participants (LCAM or LCAS), comparisons were performed as T2 versus AI, T3 versus AI, T4 versus AI or T2 versus RLC, T3 versus RLC and so on. In heat maps, the transcriptome signatures were plotted for all categories, including pre-pandemic healthy controls.
For cohort 3, the LC-MO signature was checked in CD14+ monocytes of each individual with LC. Participants were further grouped into Resp-PASC-BHR (N = 3) and Resp-PASC (N = 5) groups based on their pulmonary function test results, as shown in Supplementary Table 3.
DGE analysis per cell type
For each of the major cell types (CD14+ monocytes, CD16+ monocytes, CD8+ T cells, NK cells, CD4+ T cells and B cells), DGE analysis was performed for LC samples separately for mild/moderate and severe samples. Comparisons were made against acute COVID-19 samples and against recovered samples using Seurat FindMarkers. Genes upregulated and downregulated in these comparisons with an adjusted P value of <0.05 and log2 (fold change) of >0.8 were considered for each cell-type analysis.
Pathway analysis per cell type
Pseudobulk of each donor at each time point was calculated, followed by similar comparisons as described in the previous section using DESeq2. GSEA using Hallmark and REACTOME pathways as background was performed using the fgsea R package. Furthermore, immune-related pathways that showed statistical significance in any comparisons were plotted. The whole list of statistically significant pathways resulting from all comparisons is shown in Source Data Fig. 2a and Extended Data Fig. 2a,b.
Pathway correlations with clinical parameters
For each sample and cell type (CD14+ monocytes, CD8+ T cells and NK cells), we computed pseudobulk gene expression profiles. Subsequently, AUC scores for the selected upregulated immune pathways, as described in Fig. 2c, were calculated in each sample. The AUC scores of these pathways were then correlated with clinical parameters using Spearman correlations. Only significant correlations were plotted.
Cytokine data analysis
Cytokine measurements for each measured cytokine were log2 transformed. Comparisons and statistical tests against COVID-naive healthy controls for each measured cytokine were performed. The Spearman correlation test was performed to assess the correlation between transformed cytokine measurements and clinical parameters.
Subclustering analysis of CD8+ T cells, NK cells and monocytes
CD8+ T cells, NK cells and CD14+ monocytes were subsetted separately and reanalyzed. Libraries contributing less than 60 cells were removed, and integration was performed using ‘RPCAIntegration’ and k.weight as 60. The top 10 principal components were used for UMAP and FindNeighbors calculation. For clustering CD8+ T and NK cells, a resolution of 0.4 was used. For CD14+ monocytes, a resolution of 0.2 was used. An AUC score for Hallmark pathways enriched in pseudobulk analysis was calculated for each cell using the AUCell R package and raw counts of each cell.
Neighborhood enrichment analysis
MiloR56 was used for differential neighborhood analysis. The kNNGraph and neighborhoods were calculated with k = 50 and d = 50. The design matrix included the sampleID, severity_timePoint and recovered or not as covariates. Differential neighborhood tests were calculated for LCAM samples (T2, T3, T4 and T5) from different time points against acute COVID-19 samples (AI) or against RLC. The resulting differential neighborhoods were annotated based on cell clusters previously obtained for each cell subset. Neighborhoods with a spatial FDR of <0.1 were considered significant.
Pseudotime and trajectory analysis
A Seurat RNA assay of CD14+ monocytes was used to create a singlecell experiment object using scater. Diffusion maps were calculated using the destiny R package57. Average dimensionality was calculated using the find_sigmas function with logCounts of single-cell data. DiffusionMap was calculated using 40 principal components and sigmas calculated in the previous step. The top diffusion components (DCs) were inspected, and DC1 and DC3 were used because DC2 showed sample-dependant bias. Slingshot58 was used for trajectory calculations. Clustering was calculated using the top 15 DCs and the Mclust package. Clusters with >90% of cells from COVID-naive healthy controls were chosen as the starting clusters for trajectory calculation, resulting in three lineages. The expression of genes involved in key pathways (from pathway enrichment analysis) and selected upregulated genes from the MC4 cluster were plotted against pseudotime values of each cell. Similarly, AUC scores of key pathways calculated per cell were plotted against pseudotime.
Peak calling and peak-to-gene linkage
Peaks were called for each major cell subset as identified from RNA-based annotations using Macs3 and Ensembl.Db.Hsapiens.v86. Peaks were linked to RNA assay-based gene expression using the LinkPeaks command. Differential peaks within each cluster were calculated using Seurat function FindMarkers with the ‘LR’ test and nCount_peaks as the latent.variable.
Transcription factor motif annotation and enrichment
The Jaspar2020 database was used as background for the motif matrix using only the human-specific motif collection. Chromvar was used to calculate transcription factor activity for each cell. Differential transcription factor motif activity for any comparison was calculated using FindMarkers with mean.fxn set to ‘rowMeans’. Motif enrichment was assessed by correcting for background peaks using MatchRegionStats.
Analysis of scRNA-seq datasets (cohort 3)
Once the donor for each cell was assigned after demultiplexing, the doublets were filtered out, and singlets were kept. Seurat version 5.0 was used for downstream analysis. The following filtration criteria were used: ‘nCount_RNA < 8,000 and nFeature_RNA < 3,500 and mitochondrial percentage < 20’. RNA integration across libraries was performed using ‘RPCAIntegration’ and the top 30 dimensions for both clustering and UMAP generation. Multiple resolutions varying from 0.2 to 0.5 were carried out to obtain clusters, and canonical markers were used for identifying cell subsets (using a combination of known markers and those used by Azimuth celltype.l2) to identify CD14+ monocytes. Further, these cells were subsetted and reintegrated with 15 principal components, and clustering was performed with a resolution of 0.2. LC-Mo/MC4 AUC scores were calculated for each cell using raw counts.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
Single-cell multiome data were submitted to European Genome–Phenome Archive and are accessible through the following IDs: EGAS50000000142, EGAS50000000143, EGAS0000001215 and EGAS0000001216. Source data are provided with this paper.
Code availability
Scripts and code are available at github.com/CiiM-Bioinformatics-group/LongCOVID.
References
Davis, H. E. et al. Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. eClinicalMedicine 38, 101019 (2021).
McCorkell, L. et al. Patient-led research collaborative: embedding patients in the long COVID narrative. Pain Rep. 6, e913 (2021).
Hastie, C. E. et al. True prevalence of long-COVID in a nationwide, population cohort study. Nat. Commun. 14, 7892 (2023).
Nalbandian, A. et al. Post-acute COVID-19 syndrome. Nat. Med. 27, 601–615 (2021).
Archambault, P. M. et al. Post-COVID-19 condition symptoms among emergency department patients tested for SARS-CoV-2 infection. Nat. Commun. 15, 8449 (2024).
Klein, J. et al. Distinguishing features of long COVID identified through immune profiling. Nature 623, 139–148 (2023).
Su, Y. et al. Multiple early factors anticipate post-acute COVID-19 sequelae. Cell 185, 881–895 (2022).
Schultheiß, C. et al. The IL-1β, IL-6, and TNF cytokine triad is associated with post-acute sequelae of COVID-19. Cell Rep. Med. 3, 100663 (2022).
Lu, S. et al. Early biological markers of post-acute sequelae of SARS-CoV-2 infection. Nat. Commun. 15, 7466 (2024).
Phetsouphanh, C. et al. Immunological dysfunction persists for 8 months following initial mild-to-moderate SARS-CoV-2 infection. Nat. Immunol. 23, 210–216 (2022).
Wong, A. C. et al. Serotonin reduction in post-acute sequelae of viral infection. Cell 186, 4851–4867 (2023).
Altmann, D. M., Whettlock, E. M., Liu, S., Arachchillage, D. J. & Boyton, R. J. The immunology of long COVID. Nat. Rev. Immunol. 23, 618–634 (2023).
Davis, H. E., McCorkell, L., Vogel, J. M. & Topol, E. J. Long COVID: major findings, mechanisms and recommendations. Nat. Rev. Microbiol. 21, 133–146 (2023).
Greenhalgh, T., Sivan, M., Perlowski, A. & Nikolich, J. Ž. Long COVID: a clinical update. Lancet 404, 707–724 (2024).
Peluso, M. J. & Deeks, S. G. Mechanisms of long COVID and the path toward therapeutics. Cell 187, 5500–5529 (2024).
Liew, F. et al. Large-scale phenotyping of patients with long COVID post-hospitalization reveals mechanistic subtypes of disease. Nat. Immunol. 25, 607–621 (2024).
Cervia-Hasler, C. et al. Persistent complement dysregulation with signs of thromboinflammation in active long COVID. Science 383, eadg7942 (2024).
Al-Aly, Z. et al. Long COVID science, research and policy. Nat. Med. 30, 2148–2164 (2024).
Hanson, A. L. et al. Iron dysregulation and inflammatory stress erythropoiesis associates with long-term outcome of COVID-19. Nat. Immunol. 25, 471–482 (2024).
Yin, K. et al. Long COVID manifests with T cell dysregulation, inflammation and an uncoordinated adaptive immune response to SARS-CoV-2. Nat. Immunol. 25, 218–225 (2024).
A Clinical Case Definition of Post COVID-19 Condition by a Delphi Consensus (World Health Organization, 2021); https://www.who.int/publications/i/item/WHO-2019-nCoV-Post_COVID-19_condition-Clinical_case_definition-2021.1
Koczulla, A. R. et al. S1 guideline post-COVID/long-COVID. Pneumologie 75, 869–900 (2021).
Li, C. et al. Comparative single-cell analysis reveals IFN-γ as a driver of respiratory sequelae after acute COVID-19. Sci. Transl. Med. 16, eadn0136 (2024).
De Vries, J., Michielsen, H., Van Heck, G. L. & Drent, M. Measuring fatigue in sarcoidosis: the Fatigue Assessment Scale (FAS). Br. J. Health Psychol. 9, 279–291 (2004).
Drent, M., Lower, E. E. & De Vries, J. Sarcoidosis-associated fatigue. Eur. Respir. J. 40, 255–263 (2012).
Hendriks, C., Drent, M., Elfferich, M. & De Vries, J. The Fatigue Assessment Scale: quality and availability in sarcoidosis and other diseases. Curr. Opin. Pulm. Med. 24, 495–503 (2018).
Klok, F. A. et al. The post-COVID-19 functional status scale: a tool to measure functional status over time after COVID-19. Eur. Respir. J. 56, 2001494 (2020).
Jonsson, A. H. et al. Granzyme K+ CD8 T cells form a core population in inflamed human tissue. Sci. Transl. Med. 14, eabo0686 (2022).
Terekhova, M. et al. Single-cell atlas of healthy human blood unveils age-related loss of NKG2C+GZMB−CD8+ memory T cells and accumulation of type 2 memory T cells. Immunity 56, 2836–2854 (2023).
Notarbartolo, S. et al. Integrated longitudinal immunophenotypic, transcriptional and repertoire analyses delineate immune responses in COVID-19 patients. Sci. Immunol. 6, eabg5021 (2021).
Krooss, S. A. et al. Bronchodilator response in post-COVID-19 patients undergoing pulmonary rehabilitation. Respiration 103, 622–629 (2024).
Bailey, J. I. et al. Profibrotic monocyte-derived alveolar macrophages are expanded in patients with persistent respiratory symptoms and radiographic abnormalities after COVID-19. Nat. Immunol. 25, 2097–2109 (2024).
Wendisch, D. et al. SARS-CoV-2 infection triggers profibrotic macrophage responses and lung fibrosis. Cell 184, 6243–6261 (2021).
Däbritz, J. et al. Activation-dependent cell death of human monocytes is a novel mechanism of fine-tuning inflammation and autoimmunity. Eur. J. Immunol. 46, 1997–2007 (2016).
Monguió-Tortajada, M., Franquesa, M., Sarrias, M.-R. & Borràs, F. E. Low doses of LPS exacerbate the inflammatory response and trigger death on TLR3-primed human monocytes. Cell Death Dis. 9, 499 (2018).
Schulte-Schrepping, J. et al. Severe COVID-19 is marked by a dysregulated myeloid cell compartment. Cell 182, 1419–1440 (2020).
Zhang, B. et al. Altered and allele-specific open chromatin landscape reveals epigenetic and genetic regulators of innate immunity in COVID-19. Cell Genom. 3, 100232 (2023).
Mohd Zawawi, Z. et al. Prospective roles of tumor necrosis factor-α (TNF-α) in COVID-19: prognosis, therapeutic and management. Int. J. Mol. Sci. 24, 6142 (2023).
Lan, F. et al. GZMK-expressing CD8+ T cells promote recurrent airway inflammatory diseases. Nature 638, 490–498 (2025).
Zhao, L. et al. Characteristics and functions of an atypical inflammation-associated GZMK+GZMB+CD8+ T subset in people living with HIV-1. Mol. Immunol. 173, 40–52 (2024).
Qiao, Y. et al. AP-1 is a key regulator of proinflammatory cytokine TNFα-mediated triple-negative breast cancer progression. J. Biol. Chem. 291, 5068–5079 (2016).
Talla, A. et al. Persistent serum protein signatures define an inflammatory subcategory of long COVID. Nat. Commun. 14, 3417 (2023).
Schultheiß, C. et al. Liquid biomarkers of macrophage dysregulation and circulating spike protein illustrate the biological heterogeneity in patients with post-acute sequelae of COVID-19. J. Med. Virol. 95, e28364 (2023).
Peluso, M. J. et al. Plasma markers of neurologic injury and inflammation in people with self-reported neurologic postacute sequelae of SARS-CoV-2 infection. Neurol. Neuroimmunol. Neuroinflamm. 9, e200003 (2022).
Zhao, J. et al. Plasma biomarkers for systemic inflammation in COVID-19 survivors. Proteomics Clin. Appl. 16, e2200031 (2022).
Yoshida, Y. et al. The transcription factor IRF8 activates integrin-mediated TGF-β signaling and promotes neuroinflammation. Immunity 40, 187–198 (2014).
Leask, A. Eureka! ETS a target for fibrosis! J. Cell Commun. Signal. 5, 325–326 (2011).
Ende, G. et al. TNF-α-mediated adhesion of monocytes to endothelial cells—the role of ephrinA1. J. Mol. Cell. Cardiol. 77, 125–135 (2014).
Kopfnagel, V. et al. The Hannover Unified Biobank (HUB)—centralized standardised biobanking at Hannover Medical School. Open J. Bioresour. 8, 2 (2021).
Purcell, S. et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am. J. Hum. Genet. 81, 559–575 (2007).
Fuchsberger, C., Abecasis, G. R. & Hinds, D. A. minimac2: faster genotype imputation. Bioinformatics 31, 782–784 (2015).
Taliun, D. et al. Sequencing of 53,831 diverse genomes from the NHLBI TOPMed Program. Nature 590, 290–299 (2021).
Heaton, H. et al. Souporcell: robust clustering of single-cell RNA-seq data by genotype without reference genotypes. Nat. Methods 17, 615–620 (2020).
Hao, Y. et al. Dictionary learning for integrative, multimodal and scalable single-cell analysis. Nat. Biotechnol. 42, 293–304 (2024).
Stuart, T., Srivastava, A., Madad, S., Lareau, C. A. & Satija, R. Single-cell chromatin state analysis with Signac. Nat. Methods 18, 1333–1341 (2021).
Dann, E., Henderson, N. C., Teichmann, S. A., Morgan, M. D. & Marioni, J. C. Differential abundance testing on single-cell data using k-nearest neighbor graphs. Nat. Biotechnol. 40, 245–253 (2022).
Angerer, P. et al. destiny: diffusion maps for large-scale single-cell data in R. Bioinformatics 32, 1241–1243 (2016).
Street, K. et al. Slingshot: cell lineage and pseudotime inference for single-cell transcriptomics. BMC Genomics 19, 477 (2018).
Acknowledgements
This project was supported by an ERC Starting Grant 948207 (ModVaccine) to Y.L., the COVID-19-Research Network of the state of Lower Saxony (COFONI) through funding from the Ministry of Science and Culture of Lower Saxony in Germany (14-76403-184) to Y.L. and T.I., the Lower Saxony Center for AI and Causal Methods in Medicine (CAIMed) grant (ZN4257) and German Federal Ministry of Education and Research grants (01EQ2302A, FEDCOV, 031L0318A, AID-PAIS) to Y.L. and US National Institutes of Health grants AG069264, AI147394, HL170961, AI176171 and AG090337 to J.S. S.A.K. was supported by the PRACTIS Clinician Scientist Program, funded by MHH and DFG (DFG ME 3696/3), and by funding from Julitta und Richard Müller Stiftung (COVIDCODE). The COVID-19 biobank of MHH was funded by the Lower Saxony Ministry of Science and Culture.
Funding
Open access funding provided by Helmholtz-Zentrum für Infektionsforschung GmbH (HZI).
Author information
Authors and Affiliations
Contributions
Y.L., T.I. and S.K. conceived and designed the study. I.P., S.V., D.v.W., H.L., S.A.K., I.K., A.R.K. and M.M.H. acquired clinical samples and collected clinical data. L.Z., A.A. and A.Z. generated data. S.K. and Q.Z. performed data analysis and investigation. S.K., Y.L. and S.V. coordinated project administration. B.C., A.R.M.K., H.S., D.v.W., H.L., L.P. and C.L. performed experiments. Y.L., T.I., J.S., S.H., A.R.M.K., M.W., M.C. and C.-J.X. provided resources. Y.L., M.G.N and J.S. supervised the study. Y.L. and T.I. acquired funding. S.K. and Y.L. wrote the original paper. All authors reviewed and approved the final paper.
Corresponding author
Ethics declarations
Competing interests
M.G.N. is the scientific founder of Biotrip, Salvina, TTxD and Lemba. MHH has received fees for consultations or lectures from 35Pharma, Acceleron, Actelion, Aerovate, AOP Health, Bayer, Ferrer, Gossamer, Inhibikase, Janssen, Keros, MSD and Novartis. The other authors declare no competing interests.
Peer review
Peer review information
Nature Immunology thanks Ryan Thwaites, Onur Boyman and the other, anonymous, reviewers for their contribution to the peer review of this work. Peer reviewer reports are available. Primary Handling Editor: Ioana Staicu, in collaboration with the Nature Immunology team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 LC patients show heterogenous expression of LC signature.
a, Schematic view of analysis flow for the study. b, Mean expression of LC signature genes in LCAM (n = 4) participants with longitudinal time points and NI (n = 3) and RLC (n = 3) (cohort 1).c, Mean expression of LC signature genes showing consistent expression at all timepoints d, Number of CD14+ monocytes in each category at all time points.
Extended Data Fig. 2 LCAM and LCAS show different pathways active in LC.
a, Significant GSEA pathways in LCAM at different time points compared to AIM or RLC, in B cells, CD4+ T and CD16+ monocytes b, GSEA pathways comparing LCAS at different time points from for all major cell subsets (top and bottom) (cohort 1). Pathways plotted with adj Pval < 0.1 (Kolmogorov-Smirnov-based test with permutation-derived p-values, adjusted using the Benjamini-Hochberg method). c, comparing measured cytokine levels (CCL19, IFNg, IL10, IL12p70, IL1b, IL22, IL6, IL8, IP10) in LC patients at different time points with NI (n = 33), LC: month 1.5-2.9 (n = 51), month 3-5.9 (n = 75) month 6-8.9 (n = 60) month 9-12 (n = 19) (cohort 2). Two-sided Wilcox Rank Sum Test ****: p value < 0.00001, ***: p value < 0.001, **: p value < 0.01, *: p value < 0.05, ns = not significant. Boxplot shows the median (centre), first and third quartiles(bounds) and 1.5 times the interquartile range (whiskers) d, Mean mRNA expression of TNF, CCL2 and CXCL11 across all categories (cohort 1).
Extended Data Fig. 3 TNF significantly negatively correlates with pO2 in LCAM.
a, TNF correlation with partial pressure O2 calculated separately for LCAM and LCAS (cohort 2) at month 1.5-2.9 (LCAM n = 43, LCAS n = 8), month 3-5.9 (LCAM n = 56, LCAS n = 19) and month 6-8.9 (LCAM n = 47, LCAS n = 13) b and c Correlation of all measured cytokines as for each time point against pO2. LC: month 1.5-2.9 (n = 51), month 3-5.9 (n = 75) month 6-8.9 (n = 60) month 9-12 (n = 19). Spearman correlation P values for Spearman correlation were computed using the exact/permutation-based test. The gray shaded area indicates the 95% confidence interval.
Extended Data Fig. 4 LCAM CD8+ T and NK cells show increased abundance of GZMK+ cells.
a,b, Neighbourhood enrichment analysis in LCAM (cohort 1) compared to RLC in CD8+ T and b, in NK cells. c,d, Neighbourhood abundance enrichment analysis in LCAS compared to RLC in CD8+ T and d, in NK cells. Red dots represent increased significant enrichment of neighbourhoods; blue dots represent significantly decreased enrichment. Transcriptional states showing significant enrichment with spatial FDR < 0.1 in red and blue (F-test statistic from the quali-likelihood F-test, graph weighted FDR). e, GZMK+ cells signature derived from Jonnson et.al28 shown in gene expression profile within CD8+ T sub-clusters, f, within NK sub-clusters. g, Proportion of MC4 cells in CD14+ monocytes of LCAM samples from multiple time points stratified on sex.
Extended Data Fig. 5 LC-Mo show a distinct transcriptional program.
a, Mean gene expression of MC4 signature in CD14+ monocytes of LCAM (n = 5) patients with longitudinal samples in cohort 1, with sampling day of each individual and percentage of MC4-like cells in each sample b, Differential neighbourhood enrichment in CD14+ monocyte of LCAS compared to AIS and RLC. Red dots represent increased significant enrichment of neighbourhoods; blue dots represent significantly decreased enrichment. Transcriptional states showing significant enrichment with spatial FDR < 0.1 (F-test statistic from the quali-likelihood F-test, graph weighted FDR) c, Pathway AUC scores within monocyte clusters. Two-sided Wilcox Rank Sum Test ****: pvalue < 0.00001, ***: pvalue < 0.001, **: pvalue < 0.01, *: pvalue < 0.05, NS = Non-significant d-e, Distinct trajectories of CD14+ monocytes derived from pseudotime d, Diffusion map of all CD14+ monocytes with predicted lineages (left) cells split on categories of samples (right) e, AUC scores of pathways arranged across lineage 1 and lineage 3. f, Percentage of MC4 cells within CD14+ monocytes, categorised and binned.
Extended Data Fig. 6 Flow cytometry of CD14+ monocytes shows higher expression of LC-Mo in LC patients.
a, Gating strategy implemented to identify Monocyte subsets in cohort 4. b, Histograms for the flow cytometry analysis of CALR, CD99, TGFB1, HLA-DQ, and CD120b surface expression enriched for all CD14+ monocytes from RA+NI compared to LC. RA+NI, LC antibody, and isotype-stained cells are shown in blue, red, and black, respectively. Shown is a representative donor of biological replicates with similar results c, Comparison of Median Fluorescence Intensity (MFI) of measured markers (IFNGR1, CD51, CD105, IRF8, HLA-DR, CD16 and CD163) in CD14+ monocytes. RA+NI (n = 10), LCAM (n = 29), LCAS (n = 11). P-values calculated using two-sided Wilcox Rank Sum Test * **: p value < 0.00001, ***: p value < 0.001, **: p value < 0.01, *: p value < 0.05. Boxplot shows the median (centre), first and third quartiles(bounds) and 1.5 times the interquartile range (whiskers) d, TGFB1 MFI correlation with blood pO2 (top), the gray shaded area indicates the 95% confidence interval. correlation of significant markers in LC patients among each other (bottom). Spearman correlation P values were computed using a t-distribution approximation applied to rank-transformed data. ***: p value < 0.001, **: p value < 0.01, *: p value < 0.05.
Extended Data Fig. 7 CD8+T and NK cells show persistent increased AP-1 accessibility in LCAM.
a,b, ChromVar motif accessibility enrichments (cohort 1) of AP-1 family in CD14+ monocytes of LCAM, CD8+ T cells and NK cells in comparison to RLC. Plotted TF motifs are significant with an adjusted P-value < 0.05 (two-sided Wilcox Rank Sum Test, adjusted using the Benjamini-Hochberg method) c, Coverage plot of highlighted peaks in Fig. 5 f and g, showing normalized signal in each of MC1-4 clusters.
Extended Data Fig. 8 LC-Mo like macrophages show pro-fibrotic signature.
a, Blood (cohort 5) CD14+, CD16+ Monocytes and monocyte derived DCs from independent cohort (GSE263817)23 b, Gene expression profile of clusters showing LC-Mo/MC4 like genes in cluster5. (c) Proportion of blood and BAL Monocyte/Macrophage cells in each of the cluster as described in Fig. 6d. d, Gene expression as described in (GSE263817)23 for Tissue resident Alveolar Macrophages (TRAM) and monocyte derived Macrophages(moAM) e, Gene expression profile derived from Wendisch et al. 33 of CI1-CI6 clsuters as shown in Fig. 6df, Proportion of cells in either CI4, CI5 or CI6 clusters from each donor classified into PASC categories.
Extended Data Fig. 9 LC-Mohi patients show increased FOXO family accessibility after stimulation.
a, UMAP of all cells from non-stimulated and stimulated samples (cohort 1 subset) b, Canonical markers identifying each of the cell subsets as in a c, Percentage of cell subsets per sample from both stimulated and -non-stimulated samples. d UMAP of stimulated and non-stimulated cells (Myeloid only) e, GSEA pathway enrichment in comparison of stimulated LCAM vs stimulated RLC f, Chromvar TF accessibility in LC-Mohi and LC-Molo before and after stimulation. TF motifs are significant with adj Pval < 0.05 (two-sided Wilcox Rank Sum Test, using the Benjamini-Hochberg method).
Supplementary information
Source data
Source Data Fig. 2a and Extended Data Fig. 2a,b
Complete list of pathway comparisons against NI, RLC and AI (all categories).
Source Data Fig. 5
Motif enrichments in LC-Mo chromatin-accessible regions.
Source Data Fig. 7
DGE between LC-Mohi and LC-Molo.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kumar, S., Li, C., Zhou, L. et al. A distinct monocyte transcriptional state links systemic immune dysregulation to pulmonary impairment in long COVID. Nat Immunol 27, 200–212 (2026). https://doi.org/10.1038/s41590-025-02387-1
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41590-025-02387-1
This article is cited by
-
Immunologically distinct long COVID after mild acute disease
Nature Immunology (2026)